Vertex Pharmaceuticals Bundle
How did Vertex Pharmaceuticals become a biotech powerhouse?
Embark on a journey through the Vertex Pharmaceuticals SWOT Analysis and uncover the remarkable story of Vertex Pharmaceuticals, a biopharmaceutical company that revolutionized drug development. Founded in 1989, Vertex's innovative approach to treating serious diseases has reshaped the landscape of modern medicine. From its humble beginnings, Vertex has risen to become a global leader, significantly impacting the treatment of cystic fibrosis.

The brief history of Vertex Pharmaceuticals is a compelling narrative of scientific innovation and strategic growth. Vertex Pharmaceuticals's focus on targeted drug development has propelled it to the forefront of the biopharmaceutical industry. Examining Vertex's timeline reveals key milestones, from its early research and development to its current financial performance and market cap, solidifying its position as a leader in cystic fibrosis treatment and beyond.
What is the Vertex Pharmaceuticals Founding Story?
The story of Vertex Pharmaceuticals begins in 1989. It was founded by Dr. Joshua Boger, a chemist, and Kevin J. Kinsella. Their vision was to revolutionize drug discovery through a method called rational drug design, focusing on the molecular structures of diseases to create targeted medicines.
The company's mission from the start was ambitious: to transform how serious diseases are treated. Vertex Pharmaceuticals' headquarters are located in Boston, Massachusetts. The early days of the company, and its innovative scientific approach, were chronicled in Barry Werth's 1994 book, 'The Billion-Dollar Molecule'.
Vertex Pharmaceuticals initially focused on developing treatments for viral infections, inflammatory and autoimmune disorders, and cancer. This early focus on innovation laid the groundwork for its future successes, particularly in the area of cystic fibrosis treatment.
Vertex Pharmaceuticals' founding involved a shift in drug discovery approach, aiming for precision.
- Founded in 1989 by Dr. Joshua Boger and Kevin J. Kinsella.
- Focused on rational drug design, targeting molecular structures.
- Early focus areas included viral infections, inflammatory disorders, and cancer.
- Headquartered in Boston, Massachusetts.
As of 2024, Vertex Pharmaceuticals continues to be a leading biopharmaceutical company, with a strong emphasis on research and development. For a look at how it stacks up against others in the industry, check out the Competitors Landscape of Vertex Pharmaceuticals.
Vertex Pharmaceuticals SWOT Analysis
- Complete SWOT Breakdown
- Fully Customizable
- Editable in Excel & Word
- Professional Formatting
- Investor-Ready Format

What Drove the Early Growth of Vertex Pharmaceuticals?
The early years of Vertex Pharmaceuticals, a prominent biopharmaceutical company, were marked by a strong focus on building its scientific capabilities and expanding its research pipeline. The initial product pipeline included treatments for viral infections, inflammatory and autoimmune disorders, and cancer. A significant early achievement was the development of an oral treatment for HIV protease inhibitors in the 1990s, a major advancement in HIV/AIDS treatment. This period set the stage for future growth and innovation in the drug development sector.
A key strategic shift for Vertex Pharmaceuticals occurred in the early 2000s, with the company concentrating its efforts on cystic fibrosis (CF). This decision led to the development of several CF treatments that significantly improved patient outcomes, establishing Vertex as a leader in this field. This strategic focus on cystic fibrosis treatment proved to be a pivotal moment in the company's history.
By 2009, Vertex had grown to approximately 1,800 employees, with about 1,200 based in the Boston area. The company expanded its operations, establishing research facilities in San Diego, California, and Milton Park, Oxfordshire, England, in addition to its Boston headquarters. In 2011, Vertex signed a 15-year lease for two buildings at Fan Pier in Boston, a deal valued at $1.1 billion, to consolidate its headquarters and operations. This expansion was crucial for supporting its growing workforce and research activities.
Early product launches were critical for Vertex Pharmaceuticals. INCIVEK (telaprevir) was approved by the U.S. FDA in May 2011 for hepatitis C, and Kalydeco (VX-770) received FDA approval for cystic fibrosis in January 2012. These milestones highlighted the company's drug development capabilities and its commitment to addressing unmet medical needs. For more insights into the company's target market, consider reading about the Target Market of Vertex Pharmaceuticals.
This early growth phase laid the groundwork for Vertex's subsequent successes and current market position. The focus on research and development, coupled with strategic decisions like the pivot to cystic fibrosis, positioned Vertex Pharmaceuticals for long-term growth. The company's early investments in infrastructure and talent were also crucial for supporting its expanding pipeline and market presence.
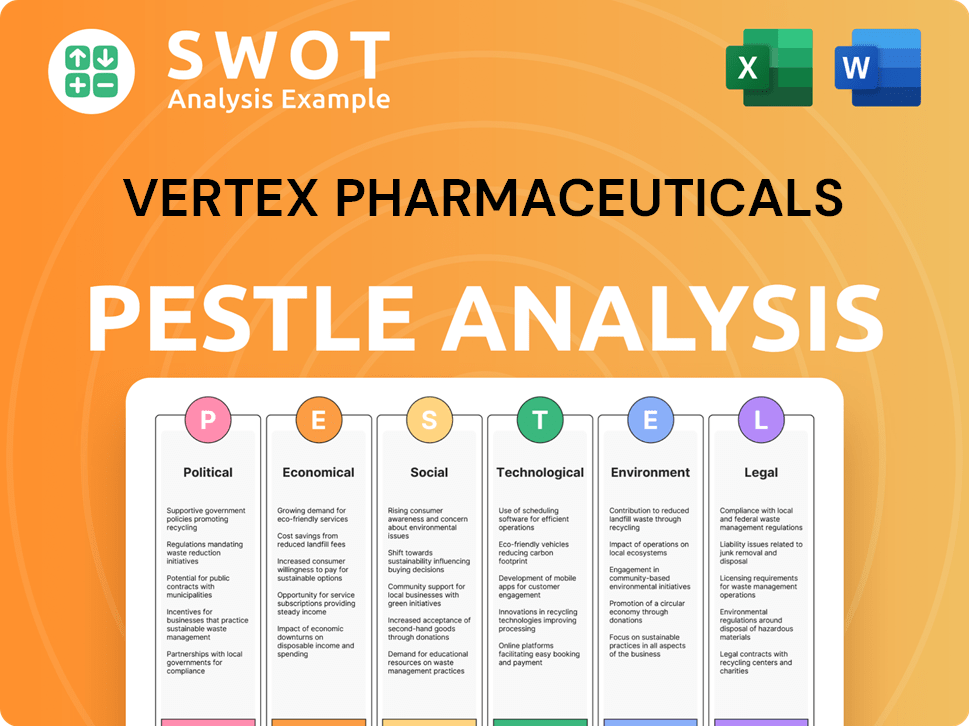
Vertex Pharmaceuticals PESTLE Analysis
- Covers All 6 PESTLE Categories
- No Research Needed – Save Hours of Work
- Built by Experts, Trusted by Consultants
- Instant Download, Ready to Use
- 100% Editable, Fully Customizable
What are the key Milestones in Vertex Pharmaceuticals history?
The Vertex Pharmaceuticals has achieved significant milestones in the biopharmaceutical industry, particularly in drug development and cystic fibrosis treatment. Its history is marked by groundbreaking advancements, strategic product launches, and expansions into new therapeutic areas, solidifying its position as a leader in the field.
| Year | Milestone |
|---|---|
| 2012 | Approval of Kalydeco (ivacaftor), the first CFTR modulator, marking a significant advancement in cystic fibrosis treatment. |
| 2019 | Launch of Trikafta/Kaftrio (elexacaftor/tezacaftor/ivacaftor), a highly effective triple combination therapy for cystic fibrosis. |
| 2024 | FDA approval for the expanded use of Trikafta, making it accessible to approximately 300 more people in the U.S. |
| 2024 | Acquisition of Alpine Immune Sciences for $4.9 billion to bolster its pipeline in APOL1-mediated kidney disease (AMKD). |
| 2025 | FDA approval for JOURNAVX™ (suzetrigine), a novel non-opioid medicine for moderate-to-severe acute pain. |
Vertex Pharmaceuticals has been at the forefront of innovation, especially in cystic fibrosis treatment. The company's development of CFTR modulators has revolutionized how the disease is managed, and its expansion into gene editing with CASGEVY for sickle cell disease and transfusion-dependent beta thalassemia shows its commitment to cutting-edge therapies.
Vertex Pharmaceuticals developed CFTR modulators to treat the underlying cause of cystic fibrosis. These drugs, including Kalydeco, Orkambi, Symdeko/Symkevi, and Trikafta, have significantly improved the lives of patients.
Trikafta/Kaftrio, a triple combination therapy, has been a major breakthrough in cystic fibrosis treatment. This has driven substantial revenue growth, with product revenue reaching $11.02 billion in 2024.
CASGEVY, a CRISPR/Cas9 gene-edited therapy, represents a significant innovation for sickle cell disease and transfusion-dependent beta thalassemia. As of late 2024, over 50 authorized treatment centers globally were activated for CASGEVY.
JOURNAVX™ (suzetrigine) is a novel non-opioid medicine for moderate-to-severe acute pain. Its approval marks Vertex Pharmaceuticals' entry into the pain management market.
Vertex Pharmaceuticals is expanding its pipeline through strategic acquisitions, such as the $4.9 billion acquisition of Alpine Immune Sciences in Q2 2024. This move strengthens its position in APOL1-mediated kidney disease.
Vertex Pharmaceuticals is expanding its global reach with CASGEVY, activating over 50 authorized treatment centers worldwide. This expansion ensures that more patients have access to innovative therapies.
Despite its successes, Vertex Pharmaceuticals faces challenges, including high costs associated with gene therapies like CASGEVY, which is priced at $2.2 million in the U.S. The company also has to navigate competitive markets and market downturns, which can impact its financial performance.
The high cost of gene therapies like CASGEVY presents significant reimbursement hurdles. This necessitates complex negotiations with payers and the exploration of new payment models.
Vertex Pharmaceuticals faces competition in new therapeutic areas. Replicating its cystic fibrosis success in crowded markets with established competitors is challenging.
Some clinical trial results have been disappointing, such as the Phase 2b trial for suzetrigine in painful lumbosacral radiculopathy (LSR) in December 2024. This highlights the risks in drug development.
Vertex Pharmaceuticals' financial performance is crucial for its continued success. The company must manage costs and navigate market fluctuations to maintain its growth trajectory.
The company faces risks associated with its pipeline, including the potential for clinical trial failures. Successful drug development is essential for long-term growth.
Strategic acquisitions, such as the purchase of Alpine Immune Sciences, are crucial for expanding Vertex Pharmaceuticals' pipeline. These moves help mitigate risks and diversify the company's portfolio.
Vertex Pharmaceuticals Business Model Canvas
- Complete 9-Block Business Model Canvas
- Effortlessly Communicate Your Business Strategy
- Investor-Ready BMC Format
- 100% Editable and Customizable
- Clear and Structured Layout

What is the Timeline of Key Events for Vertex Pharmaceuticals?
The Vertex Pharmaceuticals history began in 1989 when Joshua Boger and Kevin J. Kinsella founded the biopharmaceutical company in Massachusetts. Over the years, Vertex has marked several key milestones, including pioneering oral treatments for HIV, shifting its focus to cystic fibrosis (CF) research, and securing multiple FDA approvals for life-changing drugs. The company has expanded its capabilities through acquisitions and strategic partnerships, demonstrating a commitment to innovation and growth in the drug development sector. Vertex Pharmaceuticals' journey is a testament to its evolution and dedication to addressing serious diseases.
| Year | Key Event |
|---|---|
| 1989 | Vertex Pharmaceuticals was founded by Joshua Boger and Kevin J. Kinsella in Massachusetts. |
| 1990s | Vertex developed the first oral treatment for HIV protease inhibitors. |
| Early 2000s | The company strategically shifted its focus towards cystic fibrosis (CF) research. |
| May 2011 | The U.S. FDA approved INCIVEK (telaprevir) for hepatitis C. |
| January 2012 | The U.S. FDA approved Kalydeco (VX-770) for cystic fibrosis. |
| Mid-2014 | Vertex consolidated its headquarters and operations in new Boston facilities at Fan Pier. |
| 2019 | The company acquired Semma Therapeutics, expanding its capabilities in stem cell treatments and gene therapy. |
| December 20, 2024 | The U.S. FDA approved ALYFTREK™ (vanzacaftor/tezacaftor/deutivacaftor) for CF patients aged 6 and older. |
| December 20, 2024 | The U.S. FDA approved expanded use of TRIKAFTA for 94 additional non-F508del CFTR mutations. |
| December 31, 2024 | Vertex received regulatory approval for CASGEVY in the United Arab Emirates (UAE) for sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). |
| January 2025 | The U.S. FDA approved JOURNAVX™ (suzetrigine) for moderate-to-severe acute pain. |
| May 5, 2025 | Vertex reported Q1 2025 financial results, with total revenue of $2.77 billion. |
Vertex Pharmaceuticals is committed to diversifying its revenue base, R&D pipeline, and geographic footprint. The company projects a full-year 2025 total revenue guidance between $11.85 billion and $12.0 billion, driven by continued growth in CF and the global uptake of CASGEVY and JOURNAVX. This indicates a strong financial outlook, supported by its existing portfolio and new product launches.
Vertex has a robust pipeline with four programs in pivotal development, including povetacicept for IgA nephropathy (IgAN) and zimislecel for type 1 diabetes. The inaxaplin Phase 3 interim analysis cohort for APOL1-mediated kidney disease (AMKD) is on track to complete enrollment in the second half of 2025. Vertex plans to initiate a pivotal study of povetacicept in primary membranous nephropathy (pMN) in 2025.
Analysts generally hold a 'Moderate Buy' consensus for VRTX stock. The median price target is $512.50, implying a 14.6% upside from its current trading price of $447.09 as of May 2025. This positive outlook reflects the company's strong growth prospects and industry-leading position in the biopharmaceutical market.
Leadership emphasizes continued investment in multiple mid- and late-stage clinical development programs and global commercial and manufacturing capabilities. Vertex's future trajectory is focused on solidifying its leadership in CF while expanding into new, high-need therapeutic areas, consistent with its founding vision of transforming the treatment of serious diseases. This strategy supports long-term growth and innovation.
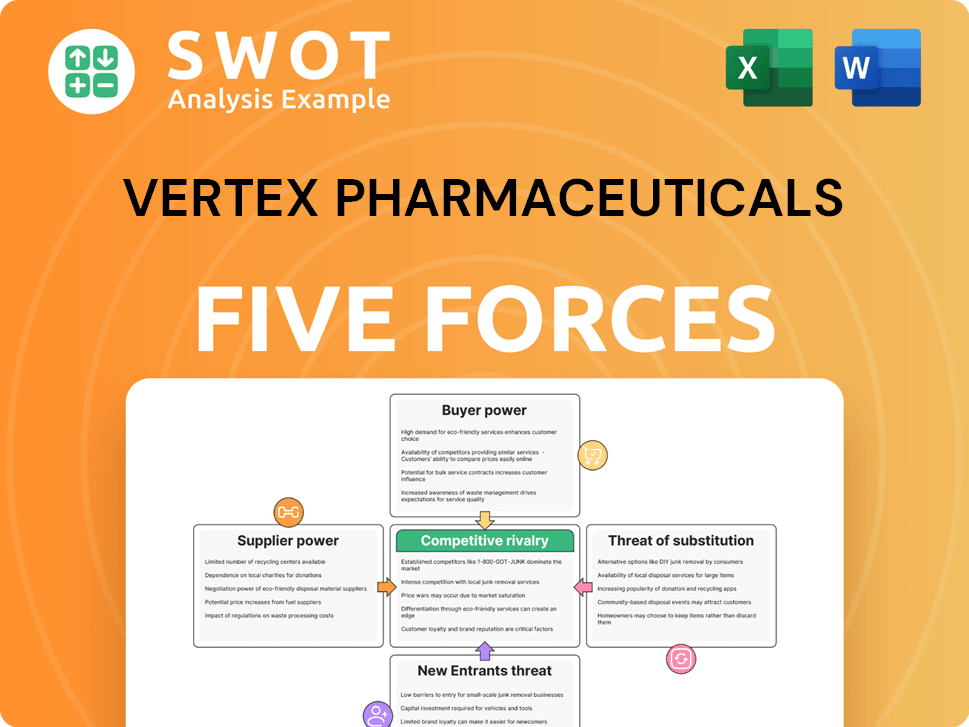
Vertex Pharmaceuticals Porter's Five Forces Analysis
- Covers All 5 Competitive Forces in Detail
- Structured for Consultants, Students, and Founders
- 100% Editable in Microsoft Word & Excel
- Instant Digital Download – Use Immediately
- Compatible with Mac & PC – Fully Unlocked
Related Blogs
- What is Competitive Landscape of Vertex Pharmaceuticals Company?
- What is Growth Strategy and Future Prospects of Vertex Pharmaceuticals Company?
- How Does Vertex Pharmaceuticals Company Work?
- What is Sales and Marketing Strategy of Vertex Pharmaceuticals Company?
- What is Brief History of Vertex Pharmaceuticals Company?
- Who Owns Vertex Pharmaceuticals Company?
- What is Customer Demographics and Target Market of Vertex Pharmaceuticals Company?
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.