Eyebright Medical Technology Bundle
How has Eyebright Medical Technology Revolutionized Eye Care?
Founded in 2010, Eyebright Medical Technology, a Eyebright Medical Technology SWOT Analysis, has rapidly become a key player in the global eye care industry. This medical technology company, based in Beijing, China, has consistently focused on innovation to improve eye health. Their journey showcases a compelling story of technological advancement and market impact, making them a fascinating case study in healthcare technology.

From its early products to its recent NMPA approval of the Loong Crystal PR phakic intraocular lens in January 2025, the company timeline reveals a commitment to medical device innovation. Eyebright Medical Technology's impact on eye care is evident as it challenges established players and capitalizes on the projected growth of the ophthalmic devices market. Understanding the brief history of Eyebright Medical Technology's founding and its major milestones is crucial for investors and industry observers alike.
What is the Eyebright Medical Technology Founding Story?
The Marketing Strategy of Eyebright Medical Technology, a medical technology company, has a compelling founding story that began in 2010. It was established by Jiangbing Xie, who returned to China after working at Abbott Laboratories. His vision was to create a company focused on medical device innovation.
The company, known as Eyebright Medical Technology (Beijing) Co., Ltd., set up its headquarters in the Science and Technology Park, Changping District, Beijing, China. The name 'Eyebright' reflects its commitment to ophthalmology and its mission of improving eye health. Eyebright's initial business model focused on the research, development, manufacturing, and sales of ophthalmic medical devices.
Eyebright's early focus was on products for eye examinations, diagnosis, and treatment. The company secured investments from Fidelity International (Eight Road), 3E Bioventures, and Tuspark Venture Capitals. A major milestone in the Eyebright history was its Initial Public Offering (IPO) on the Shanghai Stock Exchange STAR Market in July 2020, where it raised approximately RMB 882 million (US$121.29 million).
Here's a look at some important moments in the company's timeline:
- 2010: Eyebright Medical Technology was founded by Jiangbing Xie.
- Early Funding: Secured investments from Fidelity International (Eight Road), 3E Bioventures, and Tuspark Venture Capitals.
- July 2020: Eyebright's IPO on the Shanghai Stock Exchange STAR Market, raising approximately RMB 882 million (US$121.29 million). The share price surged over 500% on its first trading day.
- Focus: The company's primary objective has been to enhance eye health through continuous technological innovation and medical device innovation.
Eyebright Medical Technology SWOT Analysis
- Complete SWOT Breakdown
- Fully Customizable
- Editable in Excel & Word
- Professional Formatting
- Investor-Ready Format

What Drove the Early Growth of Eyebright Medical Technology?
The early growth of Eyebright Medical Technology, a medical technology company, focused on expanding its product portfolio and market reach. This expansion included strategic acquisitions and geographical diversification. The company's journey involved significant investments in research and development, leading to advancements in eye care. This approach helped Eyebright Medical Technology establish a strong presence in the medical device innovation sector.
Eyebright Medical Technology's core product categories include intraocular lenses (IOLs), orthokeratology lenses, and soft contact lenses. Early product launches included the Proming™ and Prosert™ series of intraocular lenses. Defocus frame lenses were also introduced. These products catered to cataract surgery, myopia management, and consumer vision care, contributing to the company's early success.
In the first half of 2024, the Proming™ and Prosert™ series of intraocular lenses saw a 30.20% year-on-year revenue increase. Defocus frame lenses experienced an 86.78% year-on-year revenue increase during the same period. The company reported a total operating revenue of RMB 1.41 billion in 2024, a 48.22% increase year-on-year, with a net income of RMB 0.387 billion, an increase of 27.36% year-on-year.
Eyebright pursued strategic acquisitions to expand its market influence and product offerings. In 2021, the company acquired Jiangsu Tianyan Medicine Technology Co. Ltd. to enter the soft contact lens market. This was followed by the acquisition of a controlling interest in Fujian Unicon Optical Co. Ltd. in 2023. These moves were key milestones in the Eyebright Medical Technology's history.
The acquisitions significantly boosted contact lens revenue. Contact lens revenue surged by 956.92% year-on-year to RMB 183.47 million in the first half of 2024, accounting for 26.76% of total revenue. Eyebright expanded geographically beyond its Beijing headquarters, establishing multiple subsidiaries across China and surrounding regions, including Suzhou, Hangzhou, and Hong Kong.
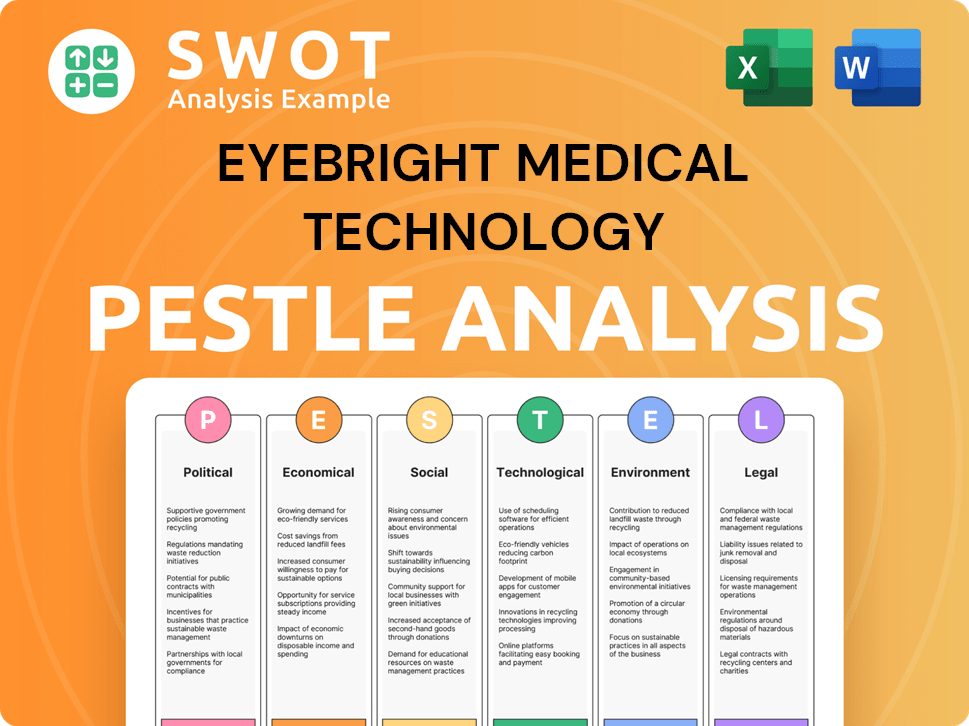
Eyebright Medical Technology PESTLE Analysis
- Covers All 6 PESTLE Categories
- No Research Needed – Save Hours of Work
- Built by Experts, Trusted by Consultants
- Instant Download, Ready to Use
- 100% Editable, Fully Customizable
What are the key Milestones in Eyebright Medical Technology history?
The Eyebright Medical Technology has achieved significant milestones, marking its journey as a prominent medical technology company. These achievements highlight the company's growth and impact on the healthcare technology sector.
| Year | Milestone |
|---|---|
| January 2025 | Received National Medical Products Administration (NMPA) approval for the Loong Crystal PR phakic intraocular lens. |
| November 2022 | Approved for China's first domestically developed multifocal intraocular lens for cataract patients. |
| October 2024 | Featured on three distinguished lists for '2024 Beijing Top 100 Enterprises' and named a 'Top 30 Value Company on the Sci-Tech Innovation Board'. |
| 2024 | Received the highest rating of Level A in the Shanghai Stock Exchange's evaluation of information disclosure for two consecutive years. |
| March 2025 | Achieved ISO 14001 certification. |
| February 2025 | Achieved ISO 50001 certification. |
Eyebright Medical Technology has consistently pushed the boundaries of medical device innovation. The company's focus on research and development has led to groundbreaking products, such as the Loong Crystal PR phakic intraocular lens, designed to correct myopia.
The Loong Crystal PR phakic intraocular lens received NMPA approval in January 2025, designed to correct myopia ranging from -3.25D to -18.00D.
In November 2022, approval was granted for China's first domestically developed multifocal intraocular lens for cataract patients, addressing a significant market need.
Despite its successes, Eyebright Medical Technology has faced challenges, including market downturns and economic conditions. However, the company has demonstrated resilience and strategic adaptability.
Economic conditions in China have impacted consumer confidence, presenting a challenge to sales and market growth.
In 2024, the company reported revenue of RMB 1,410.02 million, a 48.24% increase, and a net profit attributable to shareholders of RMB 388.40 million, up 27.77%.
A January 2025 collaboration with Meituan Health established China's first 24-hour smart contact lens stores in Beijing to boost local sales.
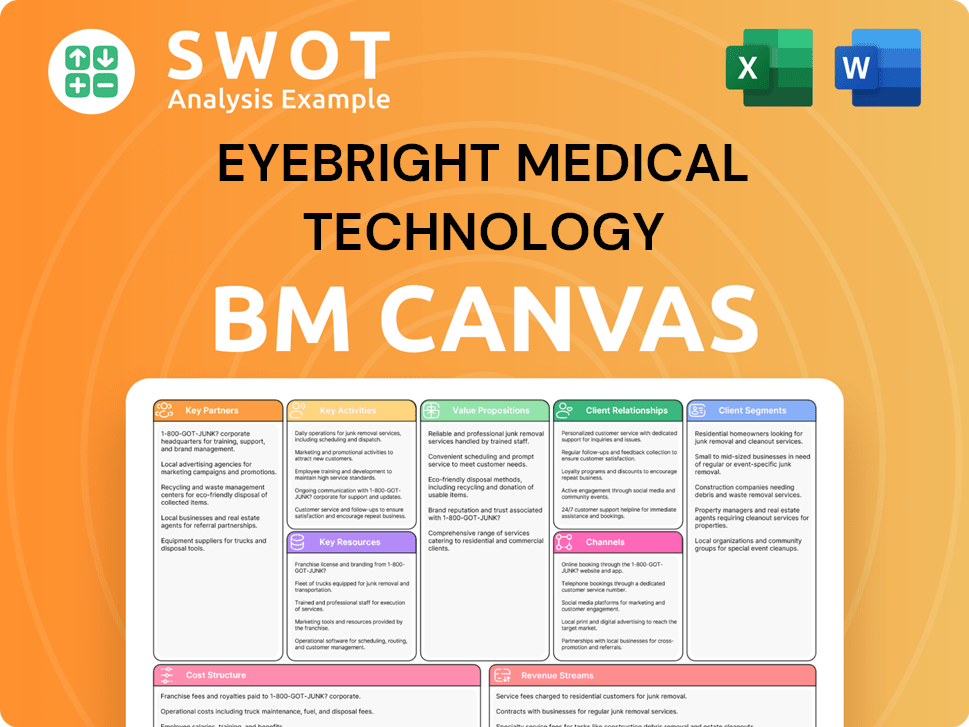
Eyebright Medical Technology Business Model Canvas
- Complete 9-Block Business Model Canvas
- Effortlessly Communicate Your Business Strategy
- Investor-Ready BMC Format
- 100% Editable and Customizable
- Clear and Structured Layout
What is the Timeline of Key Events for Eyebright Medical Technology?
The Eyebright history is marked by significant advancements in the medical technology company. The company's journey includes strategic acquisitions, innovative product launches, and expansions in the global market.
| Year | Key Event |
|---|---|
| 2010 | Eyebright Medical Technology (Beijing) Co., Ltd. was founded in Beijing, China. |
| July 29, 2020 | The company completed its Initial Public Offering (IPO) on the Shanghai Stock Exchange STAR Market, raising approximately RMB 882 million (US$114 million). |
| 2021 | Eyebright acquired Jiangsu Tianyan Medicine Technology Co. Ltd., expanding into the soft contact lens market. |
| November 2, 2022 | China approved Eyebright's first domestically developed multifocal intraocular lens for cataract patients. |
| July 28, 2023 | Eyebright agreed to acquire an additional 14.1579% stake in Fujian Unicon Optical Co., Ltd., increasing its holding to 51%. |
| 2023 | The company established its Hong Kong office, serving as its international headquarters. |
| August 22, 2024 | Eyebright Medical announced its strong first-half 2024 results, with total operating revenue reaching RMB 685.72 million, a 68.54% increase year-over-year. |
| October 24, 2024 | Eyebright was recognized as a 'Top 30 Value Company on the Sci-Tech Innovation Board'. |
| December 2024 | Eyebright Medical Technology planned to raise up to 300 million yuan by issuing 3,787,878 new shares. |
| January 3, 2025 | Eyebright Medical partnered with Meituan Health to launch China's first 24-hour smart contact lens stores in Beijing. |
| January 7, 2025 | Eyebright Medical received NMPA approval for its Loong Crystal PR phakic intraocular lens for myopia in adults. |
| February 2025 | Eyebright Medical launched the new Loong CrystalTM PR phakic intraocular lens and the iBright rigid contact lens cleaning solution at the 'Smile Vision' Academic Conference. |
| March 2025 | Eyebright achieved ISO 14001 certification for environmental management. |
| April 23, 2025 | Eyebright Medical published its 2024 Environmental, Social and Governance Report, reporting 2024 revenue of RMB 1,410.02 million (a 48.24% increase) and net profit attributable to shareholders of RMB 388.40 million (a 27.77% increase). |
Eyebright Medical Technology aims to strengthen its leading position in the ophthalmic industry. It plans to expand its product pipeline and boost medical equipment research. The company is focused on delivering world-class eye care solutions globally.
Eyebright plans to form an annual production capacity of 252 million contact lenses and 500 million pairs of molds after the completion of a fundraising investment project. This expansion will significantly increase its market reach and production capabilities.
The global ophthalmic devices market is projected to reach USD 73.74 billion by 2034, growing at a CAGR of 4.80% between 2025 and 2034. Eyebright intends to expand its market presence, particularly through its Hong Kong international headquarters.
Eyebright Medical Technology will leverage its 'intelligent manufacturing' capabilities to become a world-leading medical enterprise. This focus on technology and innovation is key to its future success. The company's vision is 'Illuminate Your World'.
Eyebright Medical Technology Porter's Five Forces Analysis
- Covers All 5 Competitive Forces in Detail
- Structured for Consultants, Students, and Founders
- 100% Editable in Microsoft Word & Excel
- Instant Digital Download – Use Immediately
- Compatible with Mac & PC – Fully Unlocked

Related Blogs
- What is Competitive Landscape of Eyebright Medical Technology Company?
- What is Growth Strategy and Future Prospects of Eyebright Medical Technology Company?
- How Does Eyebright Medical Technology Company Work?
- What is Sales and Marketing Strategy of Eyebright Medical Technology Company?
- What is Brief History of Eyebright Medical Technology Company?
- Who Owns Eyebright Medical Technology Company?
- What is Customer Demographics and Target Market of Eyebright Medical Technology Company?
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.