Eyebright Medical Technology Bundle
Can Eyebright Medical Technology Revolutionize Eye Care?
Founded in 2010, Eyebright Medical Technology, a leading Medical Technology Company based in China, is making waves in the ophthalmic medical device industry. With a focus on innovation and a recent pivotal product approval, Eyebright Medical is poised to disrupt the market. This analysis dives deep into the Eyebright Medical Technology SWOT Analysis, exploring its growth strategy and future prospects within a rapidly expanding global market.

The global Medical Device Industry is experiencing significant growth, creating a promising landscape for companies like Eyebright Medical. This article will provide a comprehensive Company Analysis, examining Eyebright Medical Technology's ambitious expansion plans, technological advancements, and financial outlook. Furthermore, we'll explore the potential challenges and opportunities that will shape its trajectory in the coming years, offering actionable insights for investors and industry professionals alike, focusing on the company's growth strategy and future market trends.
How Is Eyebright Medical Technology Expanding Its Reach?
The Growth Strategy of Eyebright Medical Technology centers on a multi-pronged approach, focusing on both expanding its market reach and diversifying its product offerings. This strategy is crucial for the Medical Technology Company to maintain its competitive edge and capitalize on the evolving demands of the Medical Device Industry. The company's initiatives are designed to foster sustainable growth and enhance its position in the market.
A key element of the expansion strategy involves international growth, particularly through its Hong Kong office, established in 2023. This strategic move leverages Hong Kong's position as a global hub and gateway to international markets, facilitating cross-border transactions and global reach. This office serves as the international headquarters, streamlining operations and enhancing global market penetration. The company's Future Prospects are significantly influenced by these strategic expansions.
Eyebright Medical Technology is also focused on expanding its product pipeline to cover the full life cycle of eye health solutions. This includes a range of intraocular lenses (IOLs) for cataract surgery, orthokeratology lenses for myopia management, and soft contact lenses, as well as lens care solutions. These expansions are strategically planned to meet the growing demands in the market and provide comprehensive eye care solutions.
The establishment of the Hong Kong office in 2023 is a pivotal step in Eyebright Medical Technology's international expansion. This office serves as the international headquarters, facilitating global operations. The company's Proming IOLs are already exported to countries including Germany, France, and Italy, demonstrating its growing international presence. This strategic move is expected to enhance the company's global reach and market penetration.
Eyebright Medical Technology is broadening its product offerings to cover the full spectrum of eye health solutions. This includes IOLs, orthokeratology lenses, soft contact lenses, and lens care solutions. The diversification aims to meet the comprehensive needs of patients and capture a larger share of the market. This approach is essential for sustained Growth Strategy.
Domestically, Eyebright Medical Technology is expanding its product pipeline to cover various eye health solutions. This includes a range of intraocular lenses (IOLs) for cataract surgery, orthokeratology lenses for myopia management, and soft contact lenses, as well as lens care solutions. The company is also focused on expanding its distribution network and increasing its market share within China.
Mergers and acquisitions are a key component of Eyebright Medical Technology's expansion strategy. Acquisitions such as Jiangsu Tianyan Medicine Technology Co. Ltd. in 2021, and Fujian Unicon Optical Co. Ltd. in 2023, have enabled the company to integrate manufacturing facilities. These strategic moves have accelerated growth in the soft contact lens business and enhanced its influence in the consumer market. For more information, you can read about the Brief History of Eyebright Medical Technology.
In the first half of 2024, Eyebright Medical Technology's contact lens revenue surged by 956.92% year-on-year, reaching RMB 183.47 million, and representing 26.76% of total revenue. The defocus frame lenses also experienced an 86.78% year-on-year revenue increase in the same period. These figures highlight the company's strong performance and effective expansion strategies.
- International Headquarters: The Hong Kong office facilitates global operations and cross-border transactions.
- Product Pipeline: Expansion includes IOLs, orthokeratology lenses, and soft contact lenses.
- Acquisitions: Strategic acquisitions enhance market presence and manufacturing capabilities.
- Financial Growth: Significant revenue increases in contact lenses and defocus frame lenses demonstrate effective expansion.
Eyebright Medical Technology SWOT Analysis
- Complete SWOT Breakdown
- Fully Customizable
- Editable in Excel & Word
- Professional Formatting
- Investor-Ready Format

How Does Eyebright Medical Technology Invest in Innovation?
The growth strategy of Eyebright Medical Technology is heavily reliant on innovation and technological advancements. The company's dedication to research and development (R&D) is a key driver of its expansion within the medical device industry. This focus allows Eyebright Medical to create new products and improve existing ones, which strengthens its market position and competitiveness.
Eyebright Medical's commitment to innovation is evident in its investment in R&D, which reached RMB 68.6 million in the first half of 2024. This represents a 25.43% year-on-year increase, demonstrating the company's dedication to staying ahead in a competitive market. This commitment is further highlighted by the expansion of its R&D team, which saw a 49.47% year-on-year increase in personnel during the same period.
The company's strategic approach to innovation is also reflected in its product development and regulatory achievements. The company's focus on technological advancements and strategic partnerships, as highlighted in this Mission, Vision & Core Values of Eyebright Medical Technology, supports its long-term growth prospects.
Eyebright Medical Technology prioritizes R&D, investing RMB 68.6 million in the first half of 2024. This investment shows a 25.43% year-on-year growth, highlighting the company's commitment to innovation.
The R&D team saw significant growth, with a 49.47% year-on-year increase in personnel during the same period. This expansion supports the company's innovation efforts.
The company launched over 20 IOL models, including aspheric, multifocal, and toric lenses. Many of these are 'first of their kind' in China, demonstrating their innovative approach.
Eyebright Medical integrates artificial intelligence (AI) in ophthalmic diagnostics. This improves precision in identifying conditions like glaucoma and diabetic retinopathy.
The company received ISO 14001 certification in March 2025 and ISO 50001 certification in February 2025. This shows its dedication to sustainability and environmental protection.
Eyebright Medical received NMPA Class III certification in January 2025 for its 'Loong Crystal PR' phakic intraocular lens, a significant milestone in its product portfolio.
Eyebright Medical Technology's innovation strategy is multifaceted, encompassing product development, technological integration, and sustainability initiatives. The company's focus on R&D, as well as its strategic partnerships, is key to its future prospects in the medical device industry.
- The 'Loong Crystal PR' phakic intraocular lens, certified in January 2025, is designed for myopia correction.
- Over 20 IOL models have been launched, including advanced lens types.
- iBright Ortho-K lenses saw a 6.89% year-on-year revenue increase in the first half of 2024.
- The company operates the 'Ophthalmic Biomaterials and Clinical Technologies' Beijing Municipal Engineering Laboratory.
- The company has undertaken numerous national key R&D programs.
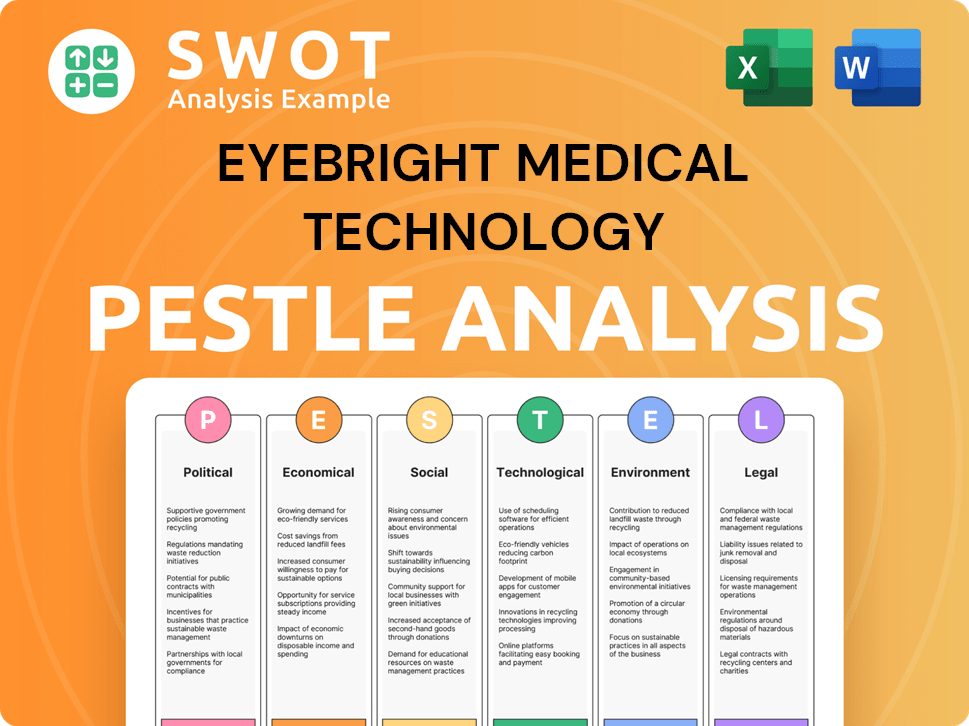
Eyebright Medical Technology PESTLE Analysis
- Covers All 6 PESTLE Categories
- No Research Needed – Save Hours of Work
- Built by Experts, Trusted by Consultants
- Instant Download, Ready to Use
- 100% Editable, Fully Customizable
What Is Eyebright Medical Technology’s Growth Forecast?
The financial outlook for Eyebright Medical Technology is robust, supported by strong revenue growth and profitability. In 2024, the company's total operating revenue reached RMB 1,410.02 million, reflecting a significant increase. This performance underscores the company's solid foundation and its potential for future expansion within the medical device industry.
Eyebright Medical Technology's financial health is further demonstrated by its net profit. The net profit attributable to shareholders in 2024 was RMB 388.40 million, with a 27.77% increase from the previous year. The company's commitment to shareholder value is evident through share buybacks and executive buy-ins, which further strengthens its financial position. For a deeper understanding, you can explore Revenue Streams & Business Model of Eyebright Medical Technology.
As of March 31, 2025, the company's profit climbed by 28%, and operating income jumped by 48%, indicating strong momentum. The trailing 12-month revenue as of March 31, 2025, was $202 million. Eyebright Medical Technology's financial performance, coupled with its strategic initiatives, positions it well for sustained growth.
Eyebright Medical Technology experienced substantial revenue growth in 2024, with total operating revenue reaching RMB 1,410.02 million, a 48.24% increase year-over-year. This growth highlights the company's success in the medical device market and its ability to capture market share.
The company's profitability also saw significant gains. Net profit attributable to shareholders increased by 27.77% to RMB 388.40 million. Excluding non-recurring items, net income reached RMB 390.08 million, reflecting a 35.08% increase, demonstrating solid financial management.
Basic earnings per share were RMB 2.05, reflecting the company's profitability and its ability to generate value for shareholders. This metric is a key indicator of the company's financial health and its potential for future growth.
As of May 23, 2025, Eyebright's stock price was $10.42, with a market capitalization of $2.01 billion. This valuation reflects investor confidence in the company's future prospects and its position in the medical technology sector.
The book value per share for fiscal years ending December 2020 to 2024 averaged 10.13, peaking in March 2025 at 12.92. Eyebright Medical's book value per share increased in 2021 (8.84, +10.3%), 2022 (9.83, +11.1%), 2023 (11.24, +14.4%), and 2024 (12.71, +13.1%). This consistent growth in book value underscores the company's strong financial position and its ability to create value.
Eyebright Medical Technology is committed to enhancing shareholder value. In 2024, the company executed a share buyback of RMB 20.04 million and executive buy-ins of RMB 3.44 million. The Shanghai Stock Exchange recognized the Company as a model for shareholder value creation in March 2025.
The company's financial performance in the first quarter of 2025 showed continued strength, with a 28% increase in profit and a 48% jump in operating income. The trailing 12-month revenue as of March 31, 2025, was $202 million, indicating robust growth momentum.
The company's book value per share has consistently increased, demonstrating its financial stability. The average book value per share from 2020 to 2024 was 10.13, peaking at 12.92 in March 2025, reflecting the company's strong asset base and financial management.
Eyebright Medical Technology's strategic initiatives, including new product development and potential partnerships, are expected to drive future revenue growth. These initiatives are crucial for maintaining its competitive edge and expanding its market share in the medical device industry.
Eyebright Medical Technology's strong financial performance and strategic initiatives position it well within the medical device industry. The company's ability to generate revenue and maintain profitability underscores its competitive advantage and potential for future growth.
The company's future prospects are promising, supported by its strong financial performance and strategic focus. The company's continued investment in research and development, coupled with its commitment to shareholder value, is expected to drive long-term growth.
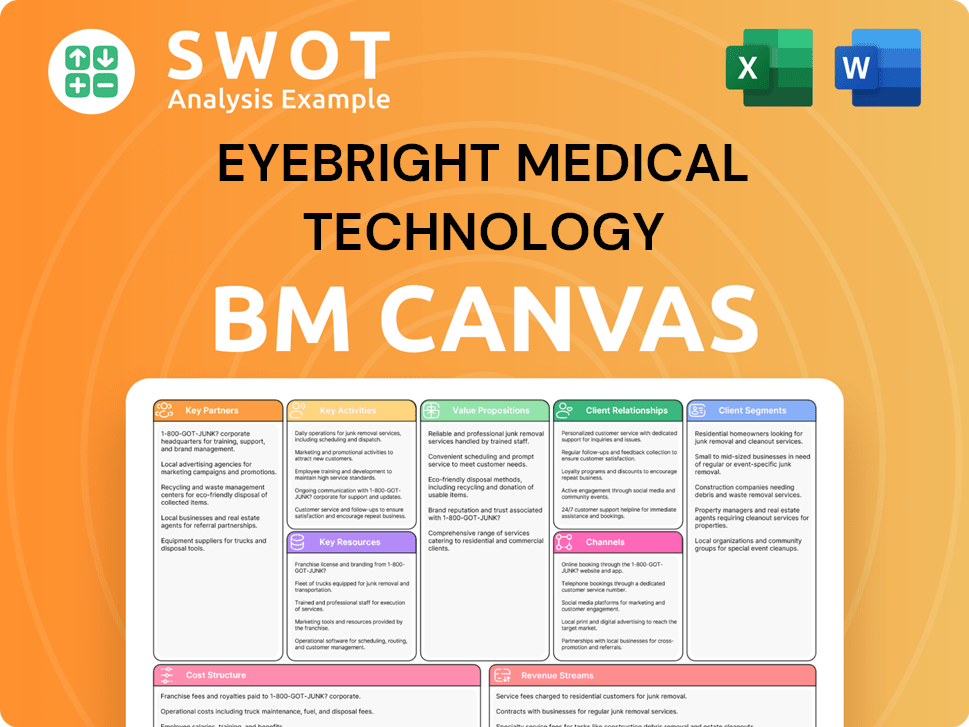
Eyebright Medical Technology Business Model Canvas
- Complete 9-Block Business Model Canvas
- Effortlessly Communicate Your Business Strategy
- Investor-Ready BMC Format
- 100% Editable and Customizable
- Clear and Structured Layout
What Risks Could Slow Eyebright Medical Technology’s Growth?
While the Eyebright Medical Technology demonstrates promising growth strategy and future prospects, several potential risks and obstacles could affect its performance within the medical technology company sector. These challenges range from intense market competition to the complexities of regulatory approvals and the need for continuous innovation. Understanding these potential pitfalls is crucial for a comprehensive company analysis.
The Medical Device Industry is inherently dynamic, and Eyebright Medical Technology must navigate a landscape shaped by rapid technological advancements, stringent regulatory requirements, and evolving market dynamics. The ability to proactively address these challenges will be vital for sustaining long-term growth and profitability. The company's success hinges on its capacity to adapt, innovate, and maintain a competitive edge.
Eyebright Medical Technology faces several significant risks that could impact its growth trajectory. These risks include intense competition, regulatory hurdles, supply chain vulnerabilities, and the need for continuous innovation. The company's ability to navigate these challenges will be critical for its long-term success.
The ophthalmic devices market is highly competitive, with significant players such as Alcon, Johnson & Johnson, Bausch + Lomb, and Carl Zeiss. The entry of new domestic and international competitors could intensify this competition. The Eyebright Medical Technology must differentiate itself to gain market share.
Obtaining and maintaining regulatory approvals, especially for Class III medical devices, poses a significant challenge. The NMPA certification process in China can take up to 18 months. Delays or failures in securing approvals could hinder product launches and revenue generation.
Supply chain disruptions, including counterfeit components and potential disruptions, are a concern. Ensuring a consistent supply of materials and components is critical. Disruptions in the supply of single-use lenses and diagnostic strips have been reported, highlighting the need for robust supply chain management.
The rapid pace of technological advancements in the medical device sector requires continuous R&D investment. Areas like AI integration and minimally invasive procedures necessitate ongoing innovation. Inaccurate health readings or software glitches could lead to product liability issues and recalls.
The need for a continuously expanding R&D team and skilled personnel could impact growth. Building and retaining a skilled workforce is essential. Internal resource constraints could limit the company's ability to capitalize on market opportunities.
The company's 'No Moat' score from GuruFocus suggests a lack of a discernible sustainable competitive advantage. This emphasizes the importance of ongoing innovation and strategic management to mitigate risks and maintain a competitive position. To learn more about Eyebright Medical Technology's competitors, read Competitors Landscape of Eyebright Medical Technology.
The ophthalmic devices market is dominated by major players. For example, Alcon reported revenues of approximately $9.8 billion in 2023. Johnson & Johnson Vision had sales of around $5.3 billion in 2023. Eyebright Medical Technology must compete effectively against these established companies.
The regulatory landscape is complex. The FDA's approval process for medical devices can take several years. In China, NMPA approval can take up to 18 months. These timelines can delay product launches and impact revenue projections.
Supply chain disruptions, as seen during the COVID-19 pandemic, can significantly impact operations. The medical device industry relies on a global supply chain. Disruptions can lead to shortages and increased costs. Ensuring supply chain resilience is crucial for maintaining production and meeting market demand.
The medical device industry is characterized by rapid technological innovation. Companies must invest heavily in R&D to stay competitive. The integration of AI and other advanced technologies requires significant capital and expertise. Failure to innovate can lead to obsolescence.
Eyebright Medical Technology Porter's Five Forces Analysis
- Covers All 5 Competitive Forces in Detail
- Structured for Consultants, Students, and Founders
- 100% Editable in Microsoft Word & Excel
- Instant Digital Download – Use Immediately
- Compatible with Mac & PC – Fully Unlocked

Related Blogs
- What are Mission Vision & Core Values of Eyebright Medical Technology Company?
- What is Competitive Landscape of Eyebright Medical Technology Company?
- How Does Eyebright Medical Technology Company Work?
- What is Sales and Marketing Strategy of Eyebright Medical Technology Company?
- What is Brief History of Eyebright Medical Technology Company?
- Who Owns Eyebright Medical Technology Company?
- What is Customer Demographics and Target Market of Eyebright Medical Technology Company?
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.