Shanghai Henlius Biotech Bundle
What's the Story Behind Shanghai Henlius Biotech's Rise?
Driven by a deeply personal mission, Shanghai Henlius Biotech, a prominent biopharmaceutical company, emerged from a vision to make life-saving drugs accessible. Founded in 2010 in Shanghai, this Shanghai Henlius Biotech SWOT Analysis offers a glimpse into its strategic evolution. From its inception, the company has focused on developing affordable biosimilars and innovative biologics, rapidly transforming the Chinese biotech industry.
Henlius Biotech's journey showcases a remarkable transformation from a startup to a global biopharmaceutical company. With a focus on oncology, autoimmune diseases, and ophthalmic diseases, Henlius Biotech's financial performance in 2024, with a 6.1% increase in revenue and a 50.3% growth in net profit, underscores its strong market position. This Biotech company overview reveals a company with a robust pipeline and a commitment to innovation and globalization, making it a key player in the Chinese biotech industry.
What is the Shanghai Henlius Biotech Founding Story?
The founding story of Shanghai Henlius Biotech, a key player in the Marketing Strategy of Shanghai Henlius Biotech, began in 2010. The company's inception was driven by a personal tragedy that fueled a vision to provide affordable, high-quality medicines, particularly in China. This commitment is reflected in the company's name, 'Henlius,' which honors a personal connection to its mission.
The company was established by Wei Dong Jiang and Shi Gao Liu, with Scott Liu Shi-kau playing a crucial role in its formation. Liu's motivation stemmed from his father's death from liver cancer in 2007, highlighting the need for accessible drugs. This experience shaped his goal to offer high-quality, affordable medicines, especially in China.
Initially, Shanghai Henlius Biotech was a joint venture between Shanghai Fosun Pharmaceutical and Henlius Biopharmaceuticals, formed in 2009. The company's primary focus was the research, development, production, and sale of monoclonal antibody (mAb) drugs, along with related consultation and technical services. Early funding included support from Fosun International. In December 2017, Shanghai Henlius Biotech raised $192.5 million by selling 55.4 million shares. Fosun contributed $50 million, securing a 62% majority stake. The company's initial focus was on developing biosimilars, bio-betters, and novel biotech drugs. Its first product, HLX01, a biosimilar to rituximab, was the first generic drug approved in China for treating non-Hodgkin lymphoma.
Shanghai Henlius Biotech was founded in 2010 by Wei Dong Jiang and Shi Gao Liu.
- The company's mission was driven by a personal tragedy, aiming to provide affordable medicines.
- Early funding included support from Fosun International.
- HLX01, a biosimilar to rituximab, was the first generic drug approved in China for non-Hodgkin lymphoma.
Shanghai Henlius Biotech SWOT Analysis
- Complete SWOT Breakdown
- Fully Customizable
- Editable in Excel & Word
- Professional Formatting
- Investor-Ready Format

What Drove the Early Growth of Shanghai Henlius Biotech?
The early growth and expansion of Shanghai Henlius Biotech, a prominent biopharmaceutical company, were marked by a strategic focus on biosimilars and innovative biologics. This Henlius Biotech company established a global footprint with R&D centers across Shanghai, Taipei, and California. Key milestones and strategic partnerships fueled its rapid development within the Chinese biotech industry.
A significant early step was the filing of an Investigational New Drug (IND) application with the NMPA for HLX01 in 2011, targeting non-Hodgkin lymphoma. This was followed by IND applications for HLX02 (breast cancer) in 2012 and HLX03 (rheumatoid arthritis) in 2013. In February 2019, Henlius launched HLX01, its first biosimilar, in accordance with China's Biosimilar Guidelines, making it the first to commercially launch such a product in China.
The approval of HANQUYOU (trastuzumab biosimilar) in China, Europe, and the U.S. further solidified its position, making it the most widely approved Chinese mAb biosimilar across multiple regions. Global clinical trials for HLX02 across China, the Philippines, and Europe were crucial for international recognition. The company's commitment to innovation and strategic partnerships has been a key factor in its success, as detailed in Growth Strategy of Shanghai Henlius Biotech.
Shanghai Henlius Biotech's revenue reached approximately RMB 5.7244 billion in 2024, a 6.1% year-over-year increase. Commercial sales of core products like HANQUYOU and HANSIZHUANG significantly contributed to this growth, with HANQUYOU's global sales reaching approximately RMB 2.8100 billion in 2024. The company has actively pursued strategic partnerships to expand its market reach and product pipeline.
In June 2022, a license agreement was formed with Organon for HLX14 (biosimilar denosumab) and HLX11 (biosimilar pertuzumab), granting Organon exclusive global commercialization rights outside of China. In February 2025, Dr Reddy's Laboratories partnered with Henlius for the development and commercialization of HLX15, an investigational daratumumab biosimilar, with Dr Reddy's gaining exclusive rights in the US and Europe. These efforts have enabled Henlius to expand its operations across Mainland China, Asia-Pacific, North America, South America, Oceania, and Europe.
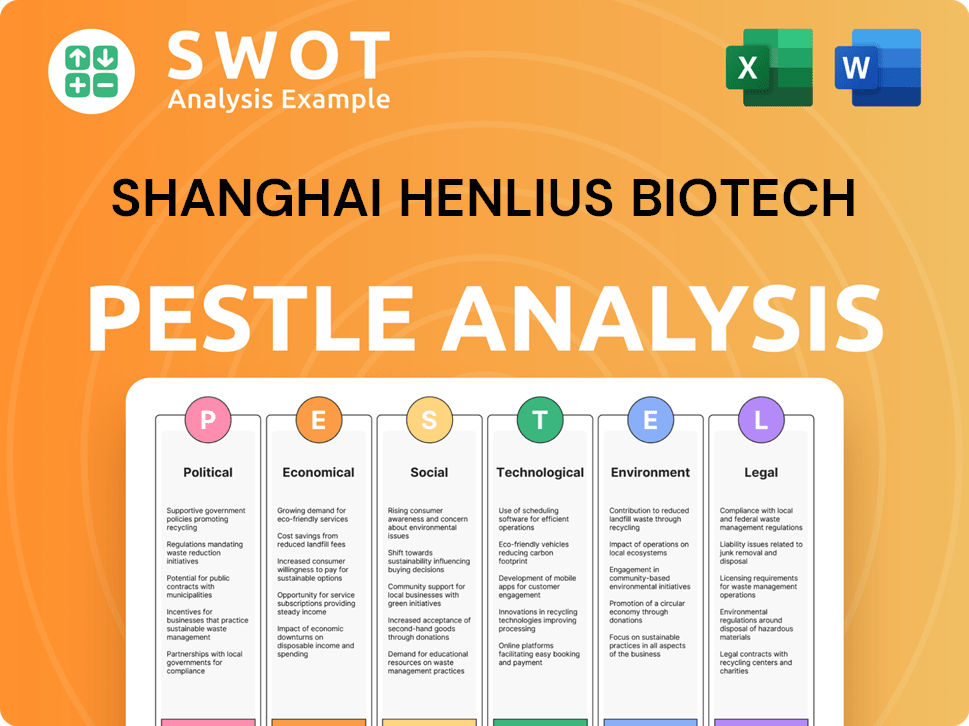
Shanghai Henlius Biotech PESTLE Analysis
- Covers All 6 PESTLE Categories
- No Research Needed – Save Hours of Work
- Built by Experts, Trusted by Consultants
- Instant Download, Ready to Use
- 100% Editable, Fully Customizable
What are the key Milestones in Shanghai Henlius Biotech history?
The Shanghai Henlius Biotech has marked several significant milestones since its inception, establishing itself as a key player in the Chinese biotech industry. These achievements highlight the company's growth and impact on the biopharmaceutical sector.
| Year | Milestone |
|---|---|
| 2019 | Approval of HANLIKANG (rituximab), China's first self-developed biosimilar. |
| 2024 | HANQUYOU (trastuzumab), a China-developed mAb biosimilar, sees global sales reach approximately RMB 2.8100 billion. |
| 2024 | HANSIZHUANG (serplulimab), the world's first anti-PD-1 mAb approved for first-line treatment of small cell lung cancer (SCLC), achieves sales of RMB 1.3126 billion. |
| 2025 | European Commission approval for HANSIZHUANG for extensive-stage small cell lung cancer (ES-SCLC). |
Henlius Biotech company has consistently pushed the boundaries of innovation, particularly in the development of biosimilars and innovative therapies. The company's focus on research and development has led to a robust pipeline of products targeting various diseases.
Approved in 2019, it was China's first self-developed biosimilar, marking a significant achievement for Henlius Biotech history.
A China-developed mAb biosimilar approved in the U.S., EU, and China, demonstrating the company's ability to meet international standards; global sales reached approximately RMB 2.8100 billion in 2024.
The world's first anti-PD-1 mAb approved for the first-line treatment of small cell lung cancer (SCLC); sales totaled RMB 1.3126 billion in 2024.
Received FDA's Orphan Drug Designation for gastric cancer in March 2025, highlighting the company's focus on innovative therapies.
The Biologic License Application (BLA) was accepted by the FDA in February 2025, indicating progress in the development of biosimilars.
The BLA was accepted by the FDA in October 2024, further expanding the company's biosimilar portfolio.
Despite its successes, Shanghai Henlius Biotech has encountered several challenges. The company faced financial losses in its early stages and competitive pressures within the biopharmaceutical sector, as well as the drug development process is lengthy and expensive with no assured outcome.
As a pre-revenue biotech, the company initially faced losses, and in 2023, there were concerns regarding the recovery of a $66.36 million investment.
Henlius Biotech company has faced competition from both domestic and international peers, impacting market share and growth.
The lengthy and expensive drug development process poses risks, with no guarantee of successful outcomes, affecting the company's financial performance.
The company's strategic pivots, including a focus on globalization and a dual-wheel strategy, have led to a transformation from a biotech to a biopharma model.
Despite the challenges, Henlius Biotech history shows the company achieving profitability for two consecutive years, with a net profit of RMB 820.5 million in 2024, demonstrating resilience.
The company has formed significant partnerships, such as the agreement with Sandoz for HLX13 (ipilimumab biosimilar) across multiple regions, potentially valued at up to $301 million.
For more insights into the competitive environment, you can explore the Competitors Landscape of Shanghai Henlius Biotech.
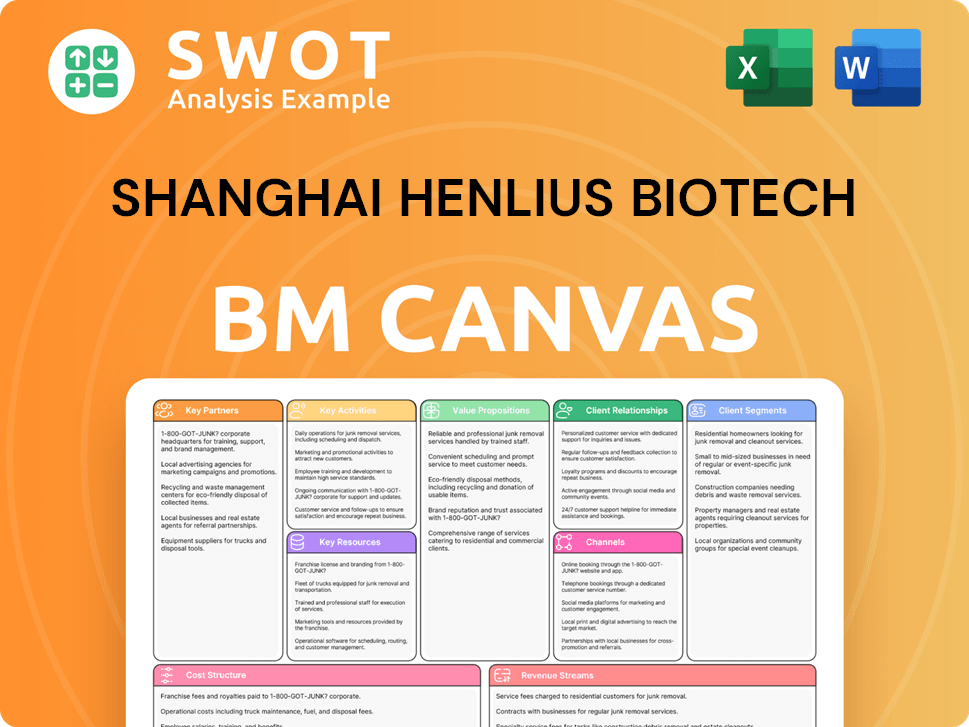
Shanghai Henlius Biotech Business Model Canvas
- Complete 9-Block Business Model Canvas
- Effortlessly Communicate Your Business Strategy
- Investor-Ready BMC Format
- 100% Editable and Customizable
- Clear and Structured Layout
What is the Timeline of Key Events for Shanghai Henlius Biotech?
The Shanghai Henlius Biotech, a biopharmaceutical company, has a rich history marked by significant milestones in the Chinese biotech industry. From its inception in 2010, the company has evolved rapidly, achieving several firsts and expanding its global presence. The company's journey includes successful product approvals, strategic partnerships, and consistent financial growth, positioning it as a key player in the development of innovative biologics.
| Year | Key Event |
|---|---|
| 2010 | Shanghai Henlius Biotech, Inc. was established in Shanghai. |
| 2011 | Filed IND application for HLX01 (non-Hodgkin lymphoma) with NMPA. |
| 2012 | Filed IND application for HLX02 (breast cancer) with NMPA. |
| 2013 | Filed IND application for HLX03 (rheumatoid arthritis) with NMPA. |
| 2017 | Raised $192.5 million in funding, with Fosun contributing $50 million. |
| February 2019 | HLX01 (rituximab biosimilar) receives NDA approval from NMPA, becoming China's first biosimilar. |
| September 2019 | Listed on the main board of the Hong Kong Stock Exchange (HKEX). |
| June 2022 | Entered into a license agreement with Organon for HLX14 (denosumab biosimilar) and HLX11 (pertuzumab biosimilar). |
| 2023 | Achieved its first full year of profitability. |
| May 2024 | IND for HLX78 (lasofoxifene) approved by NMPA; first patient dosed in Phase 3 clinical trial of HANSIZHUANG for metastatic colorectal cancer. |
| July 2024 | HANSIZHUANG approved for marketing in Thailand for ES-SCLC. |
| October 2024 | BLA for HLX14 (denosumab biosimilar) accepted by the FDA. |
| November 2024 | First patient dosed in international multi-center Phase 3 clinical study of HLX22 for gastroesophageal junction cancer and gastric cancer. |
| December 2024 | IND for Phase 1b/2 clinical trial of HLX43 (antibody-drug conjugate targeting PD-L1) approved; HANSIZHUANG approved for non-squamous non-small cell lung cancer (nsNSCLC). |
| February 2025 | Marketing Authorization Application (MAA) for HANSIZHUANG approved by the European Commission for ES-SCLC; FDA accepts BLA for HLX11 (pertuzumab biosimilar). |
| March 2025 | Orphan-drug Designation of HLX22 for gastric cancer granted by the FDA. |
| March 2025 | Announced 2024 annual results: total revenue of RMB 5.7244 billion (6.1% YoY increase), net profit of RMB 820.5 million (50.3% YoY growth). |
| April 2025 | Entered into a licensing agreement with Sandoz for HLX13 (ipilimumab biosimilar) for commercialization in the US, Europe, Japan, Canada, and Australia, with a potential deal value up to $301 million. |
Henlius is focusing on major markets, including the United States, the European Union, and Japan. The company's strategic focus is to broaden its global presence. The company plans to launch eight new products globally in the next four years.
Henlius aims to begin sales of its first product in Japan in 2027. The company has set an ambitious goal. The goal is to become a top 20 pharmaceutical company in Japan within the next decade.
The company will continue to focus on antibody drug innovation. Henlius will accelerate breakthroughs in targeted and immune therapies. The company is committed to driving the global advancement of China's innovative biologics.
Henlius plans to proactively cooperate with international partners. The aim is to facilitate marketing approval processes for its products in various countries. This approach is essential for expanding market reach.
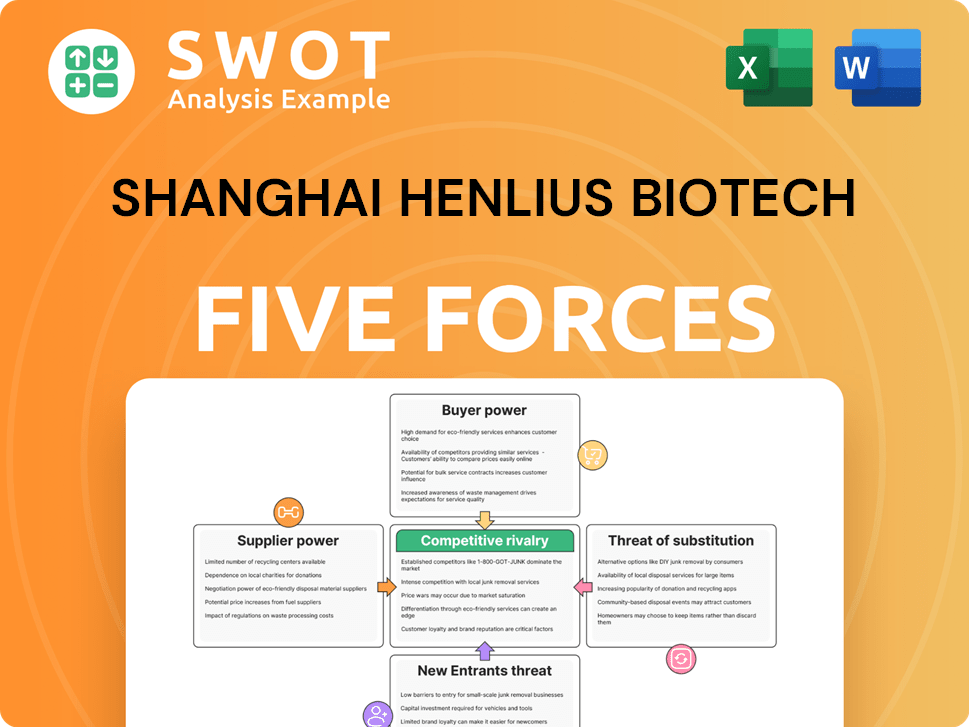
Shanghai Henlius Biotech Porter's Five Forces Analysis
- Covers All 5 Competitive Forces in Detail
- Structured for Consultants, Students, and Founders
- 100% Editable in Microsoft Word & Excel
- Instant Digital Download – Use Immediately
- Compatible with Mac & PC – Fully Unlocked
Related Blogs
- What is Competitive Landscape of Shanghai Henlius Biotech Company?
- What is Growth Strategy and Future Prospects of Shanghai Henlius Biotech Company?
- How Does Shanghai Henlius Biotech Company Work?
- What is Sales and Marketing Strategy of Shanghai Henlius Biotech Company?
- What is Brief History of Shanghai Henlius Biotech Company?
- Who Owns Shanghai Henlius Biotech Company?
- What is Customer Demographics and Target Market of Shanghai Henlius Biotech Company?
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.