Shanghai Henlius Biotech Bundle
How is Shanghai Henlius Biotech Redefining Biopharma?
Shanghai Henlius Biotech, a rising star in the biopharmaceutical world, is making waves with its focus on high-quality, affordable biosimilars and innovative biologics. In 2024, the company celebrated its second consecutive year of profitability, showcasing impressive financial growth. This success story highlights Henlius's strategic approach to drug development and its expanding global reach.
With a diverse portfolio spanning oncology, autoimmune, and ophthalmic diseases, Henlius Biotech is committed to improving patient access to vital treatments. The company's integrated platform, from research and development to commercialization, is key to its success. For a deeper dive into the company's strengths and weaknesses, consider reviewing the Shanghai Henlius Biotech SWOT Analysis, which provides valuable insights into its competitive landscape and future prospects, including its product pipeline and ongoing clinical trials.
What Are the Key Operations Driving Shanghai Henlius Biotech’s Success?
Shanghai Henlius Biotech, a prominent biopharmaceutical company, operates through an integrated platform encompassing research and development (R&D), manufacturing, and commercialization. The company focuses on creating and delivering value via a diverse pipeline of approximately 50 molecules. These include monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), and fusion proteins, targeting therapeutic areas like oncology and autoimmune diseases. Henlius's products have already benefited over 750,000 patients across more than 50 countries and regions.
The core of Henlius Biotech's operations involves streamlining processes through advanced technology, such as AI-based drug discovery. This technology has significantly reduced the time needed for drug molecular identification. The company has established global innovation centers and commercial manufacturing facilities in Shanghai, certified by Chinese, EU, and U.S. GMP standards. This ensures high-quality production and supports its R&D progress and commercial sales expansion.
Henlius leverages strategic partnerships and distribution networks to bolster its supply chain. Collaborations with global pharmaceutical companies like Sandoz and Dr. Reddy's Laboratories SA facilitate market access and commercialization in key regions. This dual strategy of 'Innovation' and 'Globalization' aims to provide high-quality, affordable biologics worldwide, increasing access to cost-effective and innovative therapies.
Henlius invests heavily in research and development, with a focus on monoclonal antibodies and other innovative therapies. The company utilizes AI-based drug discovery to accelerate the identification of drug molecules. Its R&D pipeline includes molecules in various stages of clinical trials, targeting significant unmet medical needs.
Henlius operates manufacturing facilities in Shanghai that meet stringent GMP standards. These facilities ensure high-quality production of biologics. The company's commitment to quality is crucial for gaining regulatory approvals and ensuring patient safety.
Henlius commercializes its products through strategic partnerships with global pharmaceutical companies. These collaborations expand market access and distribution networks. The company’s focus on globalization helps it reach patients in numerous countries.
Henlius offers a value proposition centered on providing high-quality, affordable biologics. This approach is supported by its robust R&D capabilities and integrated platform, leading to increased access to cost-effective therapies. The company aims to differentiate itself through a diverse and globally expanding product portfolio.
Henlius Biotech's operations are characterized by strong R&D, advanced manufacturing, and strategic partnerships. The company's focus on innovation and globalization drives its value proposition.
- R&D Pipeline: Approximately 50 molecules in various stages of development.
- Patient Reach: Products benefiting over 750,000 patients globally.
- Manufacturing: GMP-certified facilities in Shanghai.
- Partnerships: Collaborations with global pharmaceutical companies for market access. Check the Competitors Landscape of Shanghai Henlius Biotech for more information.
Shanghai Henlius Biotech SWOT Analysis
- Complete SWOT Breakdown
- Fully Customizable
- Editable in Excel & Word
- Professional Formatting
- Investor-Ready Format

How Does Shanghai Henlius Biotech Make Money?
Shanghai Henlius Biotech, a biopharmaceutical company, generates revenue through a multifaceted approach. The company's primary revenue streams include product sales, research and development (R&D) services, and license income. This diversified strategy supports its financial health and growth in the competitive pharmaceutical market.
In 2024, Henlius Biotech's total revenue reached approximately RMB 5,724.4 million, reflecting a 6.1% increase compared to RMB 5,394.9 million in 2023. Product sales are the most significant contributor, accounting for approximately RMB 4,933.5 million in 2024, which is an 8.3% year-on-year increase. This growth is driven by the successful commercialization of its key products and strategic partnerships.
Henlius Biotech also employs innovative monetization strategies, such as licensing agreements. These partnerships allow the company to expand its global reach and monetize its pipeline efficiently. This approach is crucial for the company's long-term growth and market penetration.
Several key products significantly contribute to Henlius Biotech's revenue. These products and their respective sales figures for 2024 highlight the company's market performance and product portfolio strength. The company's focus on monoclonal antibodies and drug development is evident in its product sales.
- HANQUYOU (trastuzumab): Generated approximately RMB 2.8100 billion in global sales, with domestic sales of RMB 2.6924 billion and overseas sales of RMB 117.6 million, showing a 27.0% year-on-year increase in overseas sales.
- HANSIZHUANG (serplulimab): Total sales revenue of approximately RMB 1.3126 billion, a 17.2% year-on-year increase, with domestic sales of RMB 1.3089 billion.
- HANBEITAI (bevacizumab): Approximately RMB 197.1 million in sales.
- HANNAIJIA (neratinib): Approximately RMB 45.3 million in sales.
- HANLIKANG (rituximab) and HANDAYUAN (adalimumab): Contributed RMB 550.4 million and RMB 40.1 million, respectively, through agreements with partners.
Henlius Biotech leverages strategic partnerships and licensing agreements to expand its global footprint and monetize its product pipeline. These collaborations are crucial for the company's growth and market penetration. The company's approach to partnerships is a key element of its business model, as discussed in Marketing Strategy of Shanghai Henlius Biotech.
- December 2024: Henlius granted Abbott Products Operations AG a license to commercialize five products across 69 countries and regions.
- February 2025: A licensing deal with Dr. Reddy's Laboratories SA was established for HLX15 (recombinant anti-CD38 human monoclonal antibody injection) in the U.S. and agreed European regions.
- April 2025: Henlius entered into a licensing agreement with Sandoz for its ipilimumab biosimilar HLX13, covering North America, Europe, Japan, Canada, and Australia, with Henlius eligible to receive up to $301 million, including a $31 million upfront payment.
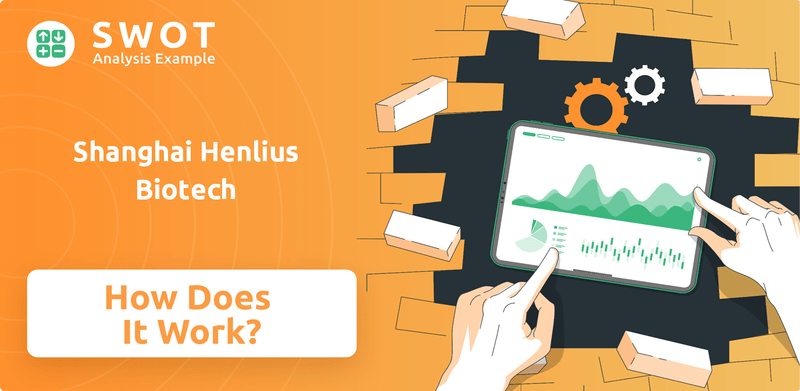
Shanghai Henlius Biotech PESTLE Analysis
- Covers All 6 PESTLE Categories
- No Research Needed – Save Hours of Work
- Built by Experts, Trusted by Consultants
- Instant Download, Ready to Use
- 100% Editable, Fully Customizable
Which Strategic Decisions Have Shaped Shanghai Henlius Biotech’s Business Model?
Shanghai Henlius Biotech, a biopharmaceutical company, has achieved significant milestones, demonstrating its growth and market presence. A key achievement was securing full-year profitability for the second consecutive year in 2024, with a net profit of RMB 820.5 million, following its first full-year profit in 2023. Product launches and approvals have been central to its growth, with a focus on monoclonal antibodies and other innovative drugs.
Strategic moves include a robust global expansion, with 25 marketing applications completed and 17 approvals secured worldwide in 2024. The company is strategically focused on major markets like the U.S., EU, and Japan, aiming for full value chain integration and deepening international partnerships. This expansion is supported by a strong research and development pipeline and a commitment to patient-centric solutions.
Despite facing operational challenges, such as weak share prices, Henlius Biotech maintains a strong position in the market. The company's competitive advantages stem from its innovation-driven R&D, with a pipeline of about 50 molecules and a focus on cutting-edge technologies. This approach allows Henlius to develop high-quality, affordable biologics, strengthening its market position.
Henlius Biotech achieved full-year profitability for the second consecutive year in 2024, with a net profit of RMB 820.5 million. This followed its first full-year profit in 2023. The company's success is driven by its focus on product launches and approvals, particularly in the area of monoclonal antibodies.
In 2024, Henlius completed 25 marketing applications and secured 17 approvals worldwide, accelerating its global presence. The company is focused on major markets like the U.S., EU, and Japan. Strategic partnerships, such as the one with SVAX in Saudi Arabia in 2024, are key to its expansion.
Henlius Biotech's competitive advantages include its innovation-driven R&D, with a pipeline of about 50 molecules. The company focuses on cutting-edge technologies like AI-based drug discovery and advanced platforms. Its ability to develop high-quality, affordable biologics strengthens its market position.
The company's financial performance is highlighted by its full-year profitability in 2024, with a net profit of RMB 820.5 million. This follows the successful commercialization of key products and strategic global expansion. The company's financial health is supported by its strong product pipeline and market approvals.
Key products like HANQUYOU (trastuzumab) and HANSIZHUANG (serplulimab) have been approved in multiple countries. In early 2025, the European Commission cleared serplulimab for extensive-stage SCLC. Henlius has a robust pipeline of about 50 molecules, focusing on innovative therapies.
- HANQUYOU (trastuzumab) is approved in China, the U.S., and Europe.
- HANSIZHUANG (serplulimab) is approved in over 30 countries and regions.
- The company is exploring targeted therapies, immunotherapies, and glyco-editing.
- Henlius is building a diversified innovation platform.
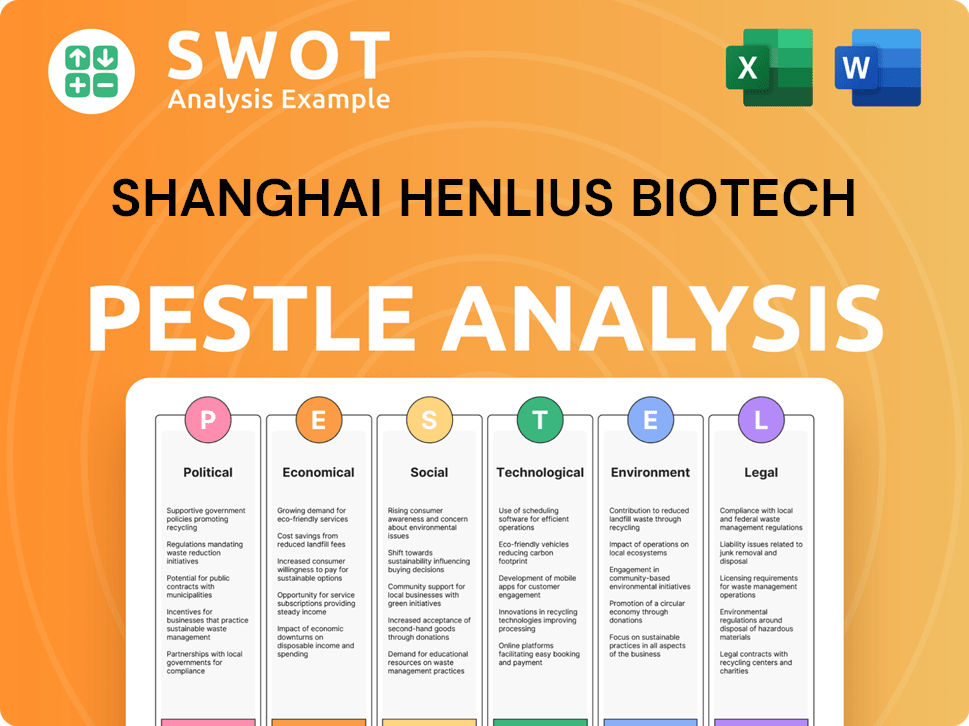
Shanghai Henlius Biotech Business Model Canvas
- Complete 9-Block Business Model Canvas
- Effortlessly Communicate Your Business Strategy
- Investor-Ready BMC Format
- 100% Editable and Customizable
- Clear and Structured Layout
How Is Shanghai Henlius Biotech Positioning Itself for Continued Success?
Shanghai Henlius Biotech has established a strong position within the biopharmaceutical industry, particularly among Chinese biotech companies. It is recognized as one of the leading emerging players in China's biotech sector. The company has a growing market share, driven by key products like HANQUYOU and HANSIZHUANG, and is one of the few Chinese biotech companies with a presence on all five continents.
The biopharmaceutical company faces several challenges, including regulatory changes, competition from established global pharmaceutical companies, and potential technological disruptions. The industry is subject to stringent regulatory approvals, and delays in clinical trials can affect revenue. Continuous innovation and differentiation are crucial for maintaining market share in a competitive landscape.
Henlius Biotech is a prominent biopharmaceutical company in China. Its products have benefited over 750,000 patients across more than 50 countries and regions. The company is expanding its global presence through its own teams and local partners.
Regulatory changes and competition from established pharmaceutical companies pose significant risks. Delays in clinical trials can impact revenue. Continuous innovation is essential to maintain market share in this dynamic industry.
Henlius plans to launch eight new products globally in the next four years. The company aims to become a top 20 pharmaceutical company in Japan by 2027. They are focused on antibody drug innovation and expanding their global commercial footprint.
The company's 2025 Global R&D Day emphasized technological advancements and international collaboration. Henlius will strengthen its production capacity and expand its global commercial footprint to deliver accessible and affordable treatment options. Learn more about the Growth Strategy of Shanghai Henlius Biotech.
Henlius is focused on accelerating breakthroughs in targeted and immune therapies. They are committed to 'Collaborate to Create' to drive growth. The company is expanding its global commercial footprint to deliver more accessible and affordable treatment options worldwide.
- Focus on antibody drug innovation.
- Expanding global commercial footprint.
- Strengthening production capacity.
- Aiming to become a top 20 pharmaceutical company in Japan by 2027.
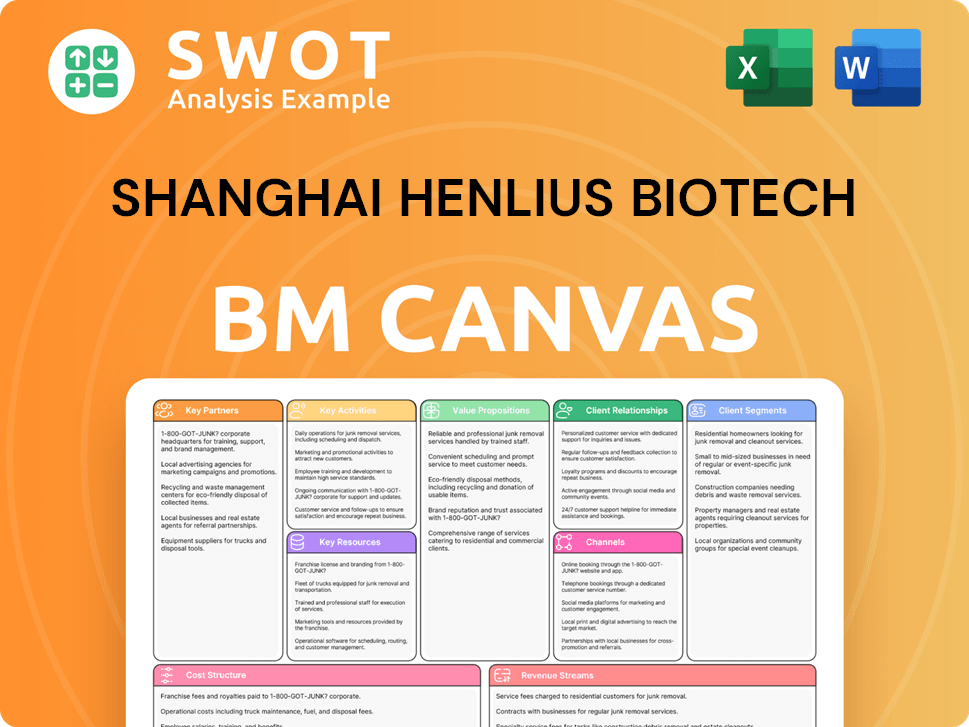
Shanghai Henlius Biotech Porter's Five Forces Analysis
- Covers All 5 Competitive Forces in Detail
- Structured for Consultants, Students, and Founders
- 100% Editable in Microsoft Word & Excel
- Instant Digital Download – Use Immediately
- Compatible with Mac & PC – Fully Unlocked
Related Blogs
- What are Mission Vision & Core Values of Shanghai Henlius Biotech Company?
- What is Competitive Landscape of Shanghai Henlius Biotech Company?
- What is Growth Strategy and Future Prospects of Shanghai Henlius Biotech Company?
- What is Sales and Marketing Strategy of Shanghai Henlius Biotech Company?
- What is Brief History of Shanghai Henlius Biotech Company?
- Who Owns Shanghai Henlius Biotech Company?
- What is Customer Demographics and Target Market of Shanghai Henlius Biotech Company?
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.