Nkarta Bundle
How Has Nkarta Company Navigated the Complex World of Biotech?
Founded in 2015, Nkarta, a clinical-stage biotech company, has charted a course through the rapidly evolving landscape of cell therapy. Initially targeting cancer with its innovative natural killer (NK) cell therapies, Nkarta's journey is a testament to strategic adaptation. This Nkarta SWOT Analysis can help you understand the company's current position.

From its inception, Nkarta's focus on harnessing the power of NK cells has been a driving force, but the company's recent pivot to autoimmune diseases showcases its agility. This strategic shift, coupled with ongoing clinical trials and a strong cash position, positions Nkarta as a company to watch in the coming years. Understanding the Nkarta history is key to appreciating its potential impact on the biotech industry.
What is the Nkarta Founding Story?
The story of the Nkarta Company begins in July 2015, when it was incorporated in Delaware. The company's main offices are in South San Francisco, California. Its roots are in the pioneering work of Dr. Dario Campana, recognized as a scientific founder.
The initial goal was to create more available and effective cell therapies. The focus was on harnessing natural killer (NK) cells. These cells have the ability to target and destroy cancer cells, but the challenge was to produce them in large enough quantities to fight the disease.
The company's early strategy centered on using NK cell expansion and cell engineering technologies. The aim was to generate a large supply of NK cells, improve their ability to recognize targets, and extend their activity. Initial product candidates were aimed at treating cancer, including NKX101 for blood cancers and solid tumors, and NKX019 for B-cell malignancies.
Nkarta's journey began with a focus on cell therapy, specifically NK cells, to combat cancer. This strategy was built on Dr. Campana's research.
- The company aimed to create 'off-the-shelf' cell therapies.
- Nkarta raised over $100 million in private funding before going public in 2020.
- A Series B financing round in September 2019 raised $114 million.
- The company planned to complete a GMP manufacturing facility in South San Francisco in 2020.
Before going public in 2020, the company secured over $100 million in private funding. A significant round was the Series B financing in September 2019, which brought in $114 million. This round was led by Samsara BioCapital and included new investors like Amgen Ventures and Deerfield Management. Existing investors such as NEA Ventures and Novo Holdings A/S also participated. A key part of their early strategy was building a GMP manufacturing facility in South San Francisco in 2020. This was to supply products for its early-stage clinical trials, showing their commitment to producing 'off-the-shelf' cell therapies. To learn more about the company's core values, you can read Mission, Vision & Core Values of Nkarta.
Nkarta SWOT Analysis
- Complete SWOT Breakdown
- Fully Customizable
- Editable in Excel & Word
- Professional Formatting
- Investor-Ready Format

What Drove the Early Growth of Nkarta?
The early growth of the Nkarta Company, a biotech company, was marked by significant developments and strategic pivots. Founded in 2015, Nkarta initially focused on developing off-the-shelf, allogeneic natural killer (NK) cell therapies, primarily for cancer treatments. Key milestones included substantial funding rounds and a public offering, which fueled the expansion of its clinical programs and manufacturing capabilities.
Nkarta's early focus was on developing NK cell therapies for cancer. The company's pipeline included NKX101, which entered Phase 1 clinical trials for relapsed/refractory acute myeloid leukemia and higher-risk myelodysplastic syndromes. Another key candidate, NKX019, was initially aimed at B-cell malignancies, targeting the CD19 antigen. This early focus laid the foundation for Nkarta's entry into the cell therapy market.
A significant boost to Nkarta's early growth was the $114 million Series B financing round in September 2019. This funding supported multiple clinical trial programs and the development of additional product candidates. In 2020, Nkarta went public, raising approximately $252.0 million in gross proceeds from its initial public offering. These financial moves were crucial for advancing the company's research and development efforts.
Nkarta aimed to complete a GMP manufacturing facility in South San Francisco in 2020 to support its early-stage clinical trials. However, the company has faced financial challenges. In 2024, Nkarta reported a net loss of about $109 million, and in the first quarter of 2025, the net loss was $32.0 million. Despite these losses, the company maintained a strong cash position.
A major strategic shift occurred in 2024 when Nkarta fully pivoted its research focus from oncology to autoimmune conditions. The company discontinued NKX019 development in lymphoma to concentrate on autoimmune diseases. This decision reflects a belief that NKX019 has greater potential to transform patient care in autoimmune diseases. The company is now evaluating NKX019 in multiple autoimmune conditions, including lupus nephritis, primary membranous nephropathy, systemic sclerosis, inflammatory myopathy, and ANCA-associated vasculitis. The Target Market of Nkarta has evolved with this shift.
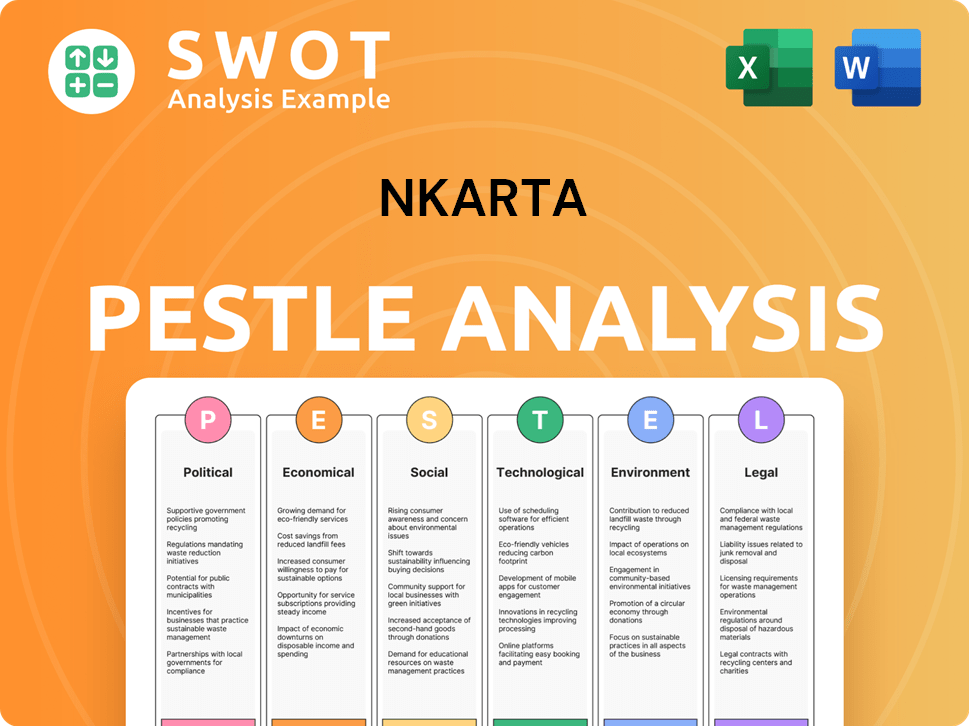
Nkarta PESTLE Analysis
- Covers All 6 PESTLE Categories
- No Research Needed – Save Hours of Work
- Built by Experts, Trusted by Consultants
- Instant Download, Ready to Use
- 100% Editable, Fully Customizable
What are the key Milestones in Nkarta history?
The Owners & Shareholders of Nkarta has experienced several pivotal moments. These milestones have shaped its trajectory within the competitive landscape of the biotech company, reflecting both its successes and the challenges it has faced in the development of cell therapy.
| Year | Milestone |
|---|---|
| September 2019 | Closed an oversubscribed Series B financing, raising $114 million to advance its programs. |
| 2020 | Went public, raising approximately $252.0 million through its initial public offering. |
| May 2021 | Announced a strategic partnership with CRISPR Therapeutics to develop CRISPR/Cas9 gene-edited cell therapies for cancer. |
| 2024 | Undertook a strategic pivot, shifting its primary focus from oncology to autoimmune diseases. |
| March 2025 | Implemented a significant restructuring plan, including a workforce reduction of 34% and a reduction of over 50% of its executive leadership team. |
A key innovation for the Nkarta Company is the development of off-the-shelf, allogeneic natural killer (NK) cell therapies. They also developed a proprietary cell expansion and cryopreservation platform to generate an abundant supply of NK cells, aiming to overcome the complexities of autologous cell therapies.
This innovation allows for readily available treatments, unlike personalized autologous therapies. This approach aims to provide a more accessible and efficient treatment option for various cancers and, more recently, autoimmune diseases.
The platform enables the generation of a large supply of NK cells. This scalable approach is crucial for meeting the demands of clinical trials and future commercialization of cell therapy products.
Nkarta has faced significant challenges, particularly in demonstrating the long-lasting benefits of its lead cancer therapy. The biotech company also struggled with a difficult funding climate, leading to a strategic pivot and restructuring in 2024 and 2025.
The cell therapy market is highly competitive, with numerous companies developing similar therapies. This competition has made it challenging to secure market share and funding for Nkarta's clinical trials.
In 2024, Nkarta experienced a net loss of approximately $109 million, highlighting the financial pressures faced by biotech companies. This financial strain has led to strategic shifts and restructuring efforts.
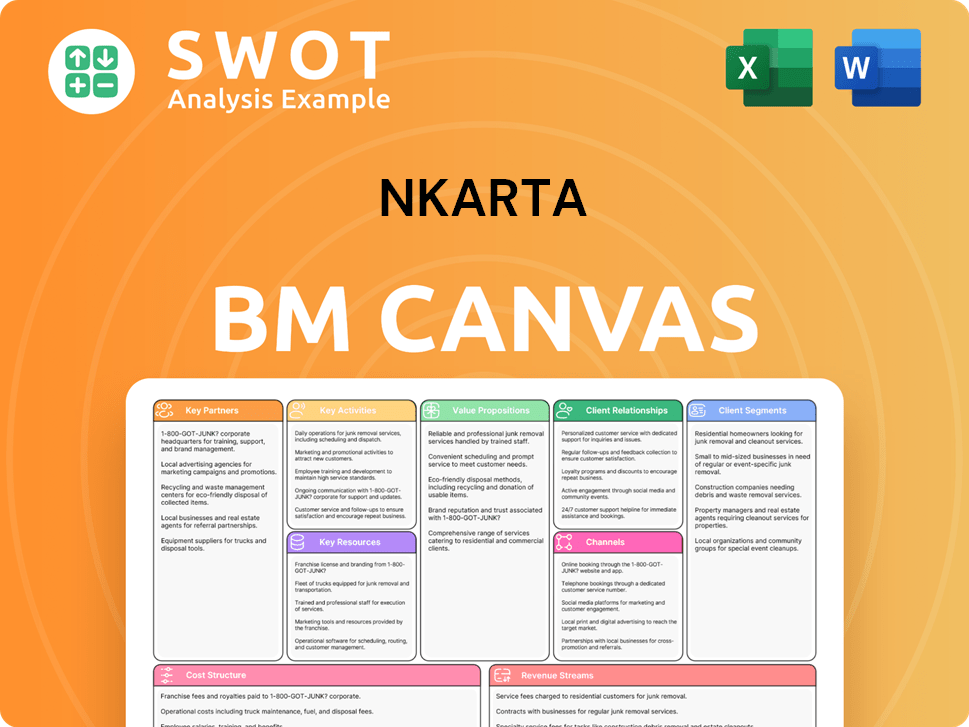
Nkarta Business Model Canvas
- Complete 9-Block Business Model Canvas
- Effortlessly Communicate Your Business Strategy
- Investor-Ready BMC Format
- 100% Editable and Customizable
- Clear and Structured Layout
What is the Timeline of Key Events for Nkarta?
The Nkarta Company's history is marked by strategic pivots and financial milestones that have shaped its current focus on cell therapy. From its incorporation in 2015 to its IPO in 2020 and the more recent restructuring in 2025, the company has navigated significant developments in the biotech sector.
| Year | Key Event |
|---|---|
| 2015 | Nkarta, Inc. was incorporated in Delaware. |
| October 2015 | Nkarta secured its first seed funding round. |
| September 2019 | Nkarta closed an oversubscribed $114 million Series B financing round. |
| 2020 | Nkarta completed a GMP manufacturing facility in South San Francisco and went public with an Initial Public Offering (IPO), raising approximately $252.0 million. |
| May 2021 | Nkarta announced a strategic partnership with CRISPR Therapeutics to develop gene-edited cell therapies for cancer. |
| Q4 2023 | Investigational New Drug (IND) application for NKX019 in lupus nephritis was cleared. |
| 2024 | Nkarta fully pivoted its research focus from oncology to autoimmune conditions, discontinuing NKX019 development in lymphoma and launched Ntrust-1 and Ntrust-2 trials for NKX019 in multiple autoimmune conditions. |
| Q4 2024 | The company reported a net loss of approximately $109 million for the full year. |
| March 2025 | Nkarta announced a strategic restructuring, including a 34% workforce reduction and over 50% reduction in executive leadership, to extend cash runway into 2029 and reported a cash balance of $351.9 million. |
| Q1 2025 | Nkarta reported a net loss of $32.0 million. |
| Second Half of 2025 | Initial clinical data from the Ntrust-1 and Ntrust-2 trials for NKX019 in autoimmune indications are expected. |
Nkarta is now primarily focused on advancing NKX019 for autoimmune diseases. The company is concentrating on demonstrating the safety and effectiveness of NKX019 through its ongoing clinical trials. Initial clinical data from the Ntrust-1 and Ntrust-2 trials, expected in the second half of 2025, will be critical.
Analysts project Nkarta's annual revenue to grow by 73.2% per year, but earnings are forecast to decline by 0.7% annually. The company is anticipated to remain unprofitable over the next three years. The 2025 restructuring aims to extend the cash runway into 2029, providing financial stability for achieving clinical milestones.
Nkarta's long-term goal is to make cell therapy more accessible by pioneering allogeneic NK cells that can be administered in outpatient settings. The company believes its treatments could offer the advantages of personalized cell therapies with the convenience of an off-the-shelf solution. The company's mission is to develop and deliver innovative cell therapies.
The strategic restructuring announced in March 2025, included a 34% workforce reduction and over 50% reduction in executive leadership. This restructuring aims to extend the company's financial runway, which is critical for supporting ongoing research and development efforts. The restructuring is expected to extend the company's cash runway into 2029.
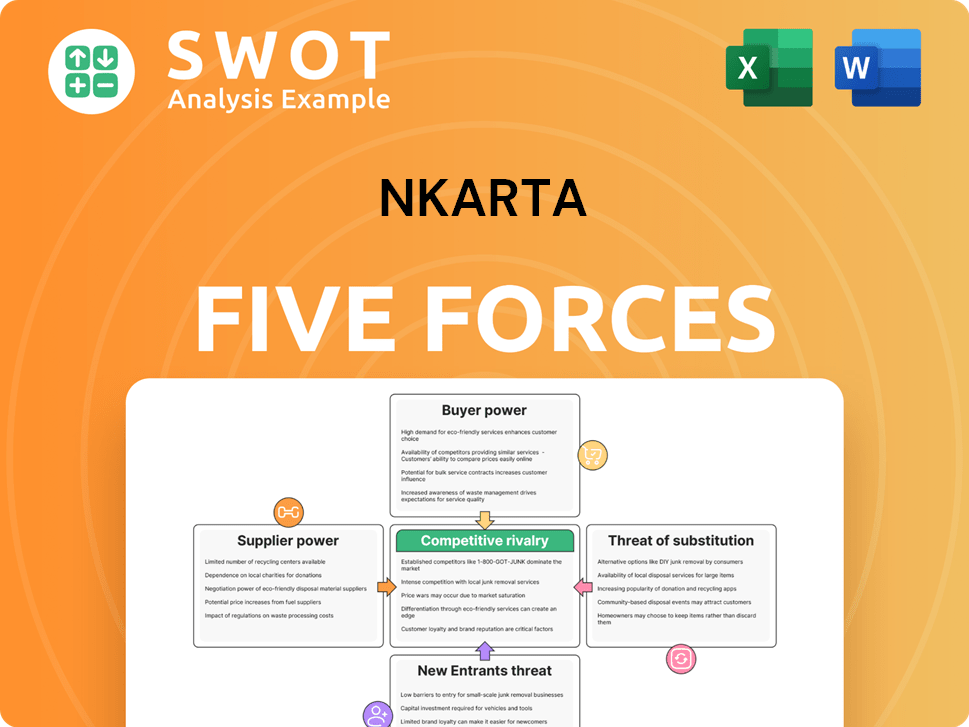
Nkarta Porter's Five Forces Analysis
- Covers All 5 Competitive Forces in Detail
- Structured for Consultants, Students, and Founders
- 100% Editable in Microsoft Word & Excel
- Instant Digital Download – Use Immediately
- Compatible with Mac & PC – Fully Unlocked
Related Blogs
- What is Competitive Landscape of Nkarta Company?
- What is Growth Strategy and Future Prospects of Nkarta Company?
- How Does Nkarta Company Work?
- What is Sales and Marketing Strategy of Nkarta Company?
- What is Brief History of Nkarta Company?
- Who Owns Nkarta Company?
- What is Customer Demographics and Target Market of Nkarta Company?
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.