Nkarta Bundle
How is Nkarta Company Redefining Its Customer Focus?
In the dynamic world of biopharmaceuticals, understanding Nkarta SWOT Analysis is essential for strategic success. Nkarta Company, a pioneer in cell therapy, has undergone a significant transformation, shifting its focus from oncology to autoimmune diseases. This strategic pivot necessitates a deep dive into the company's evolving customer demographics and target market.

This shift presents a compelling case study in market analysis, exploring how Nkarta Company adapts to new customer needs and wants. Understanding Nkarta's customer profile, including patient demographics and geographical target market, is crucial for investors and stakeholders. We'll delve into the company's customer acquisition strategy and market share analysis to assess its potential customers and navigate the competitive landscape, considering current market trends.
Who Are Nkarta’s Main Customers?
The primary customer segments for the [Company Name] center around patients grappling with severe autoimmune diseases, specifically those where B-cell mediated depletion is a viable treatment approach. This strategic focus shift became pronounced in late 2024 and early 2025, marking a significant pivot in the company's developmental strategy. This change involved discontinuing its lymphoma development efforts to concentrate on autoimmune indications, reflecting a strategic realignment towards unmet medical needs.
The company's lead product candidate, NKX019, is currently undergoing evaluation in multiple clinical trials targeting these conditions. This shift underscores a commitment to addressing the challenges faced by individuals with autoimmune diseases, aiming to provide innovative therapeutic solutions. The focus on autoimmune diseases highlights a strategic decision to concentrate resources on areas with significant unmet needs.
The company's business model is B2B, with healthcare providers and institutions as direct customers. These entities administer the complex cell therapies to patients. This approach emphasizes an 'off-the-shelf' solution designed for broad accessibility, including outpatient settings. This business model addresses significant logistical and accessibility challenges associated with existing cell therapies, providing a streamlined approach for healthcare providers.
The Ntrust-1 clinical trial for patients with refractory lupus nephritis commenced in June 2024, with patient screening underway. These patients typically receive three-dose cycles of NKX019 after lymphodepletion. Additional cycles may be administered to maintain response. This trial is a key component of the company's strategy.
The FDA clearance for the Ntrust-2 trial was received in June 2024, with enrollment anticipated to begin by the end of 2024. This trial also includes patients with idiopathic inflammatory myopathy (myositis) and ANCA-associated vasculitis (AAV). This expansion demonstrates a broader approach to autoimmune disease treatment.
These conditions are also part of the Ntrust-2 trial, reflecting a comprehensive strategy for addressing B-cell mediated autoimmune diseases. The inclusion of these diseases expands the scope of the company's clinical trials. This approach aims to provide diverse treatment options.
An investigator-sponsored trial (IST) of NKX019 for SLE, spearheaded by researchers at Columbia University Irving Medical Center, initiated in November 2024. This trial signifies the company's commitment to exploring treatments for SLE. The IST highlights collaborative research efforts.
While specific demographic data like age, gender, income, education, and occupation are not explicitly provided, the targeted autoimmune diseases often affect a wide range of individuals. These conditions can significantly impact a patient's quality of life and ability to work. This highlights the critical need for effective and accessible treatments. Understanding the Owners & Shareholders of Nkarta can provide further context on the company's strategic direction and customer focus, which is crucial for any thorough market analysis.
The shift towards autoimmune diseases was driven by a re-evaluation of the competitive landscape and strategic prioritization of NKX019. This pivot suggests the company identified a greater unmet need or a more favorable environment within the autoimmune space. This strategic realignment is crucial for understanding the company's market position and potential for growth.
- Lupus Nephritis (LN): Focus on patients with refractory disease, with trials initiated in June 2024.
- Systemic Sclerosis (SSc): Ntrust-2 trial enrollment expected to begin by the end of 2024.
- Idiopathic Inflammatory Myopathy (IIM) and ANCA-associated Vasculitis (AAV): Included in the Ntrust-2 trial, broadening the scope.
- Systemic Lupus Erythematosus (SLE): Investigator-sponsored trial (IST) initiated in November 2024.
Nkarta SWOT Analysis
- Complete SWOT Breakdown
- Fully Customizable
- Editable in Excel & Word
- Professional Formatting
- Investor-Ready Format

What Do Nkarta’s Customers Want?
Understanding the customer needs and preferences is crucial for the success of any company, and this is especially true for a biotech firm like Nkarta Company. Their target market consists of patients suffering from severe autoimmune diseases, such as lupus nephritis, systemic sclerosis, and others, who have specific requirements and desires that drive their demand for novel therapies. A deep dive into the customer demographics and the target market reveals the critical factors influencing their choices and the potential of Nkarta's offerings.
The primary need for these patients is effective treatment that goes beyond symptom management, aiming for deep and durable remission. Current treatment options often fall short, leading patients to seek therapies that offer improved efficacy with better safety profiles and accessibility. Nkarta's approach is designed to address these needs, focusing on innovative cell therapies to provide better outcomes for its target market.
The customer profile of Nkarta's target market is defined by the challenges they face with existing treatments and their aspirations for a better quality of life. The company's strategy is centered around meeting these needs, which is evident in its clinical trial designs and product development efforts. The goal is to provide therapies that are not only effective but also convenient and accessible, addressing the limitations of current treatments.
Patients want treatments that provide long-term relief and address the underlying disease. This goes beyond managing symptoms to achieving remission, allowing them to live without the constant burden of their condition.
A key preference is for therapies with fewer side effects compared to existing treatments. Nkarta's NKX019 aims to minimize toxicity, improving patient tolerability and overall well-being.
The 'off-the-shelf' availability and potential for outpatient administration are highly desirable. This contrasts with complex autologous cell therapies, making treatment more accessible and less resource-intensive.
Patients seek the hope of a better quality of life, free from the chronic burden of their disease. This includes the possibility of long-term, drug-free remissions, driving their interest in innovative therapies.
Receiving treatment in an outpatient setting reduces the burden of hospital stays and associated costs, offering a practical advantage for patients. This improves the overall treatment experience.
Nkarta directly addresses the limitations of current therapies in terms of efficacy and safety. The company focuses on creating therapies designed for deep therapeutic activity and broad access, tailored to specific patient needs.
The customer needs and preferences of patients with severe autoimmune diseases are central to Nkarta's product development and market strategy. Understanding the patient's perspective is crucial for success. The company's approach is informed by these insights, aiming to provide therapies that meet the unmet needs of this specific population. For more details, you can read about the Marketing Strategy of Nkarta.
- Efficacy and Safety: Patients prioritize treatments that are both effective in controlling their disease and safe, minimizing adverse effects.
- Accessibility: The convenience of treatment, including outpatient options, is a significant factor in patient preference, reducing the burden of treatment.
- Quality of Life: The ultimate goal is to improve the patient's quality of life, enabling them to live more normally and free from the debilitating effects of their condition.
- Long-Term Outcomes: Patients seek therapies that offer the potential for long-term remission, ideally drug-free, to avoid the chronic nature of their disease.
Nkarta PESTLE Analysis
- Covers All 6 PESTLE Categories
- No Research Needed – Save Hours of Work
- Built by Experts, Trusted by Consultants
- Instant Download, Ready to Use
- 100% Editable, Fully Customizable
Where does Nkarta operate?
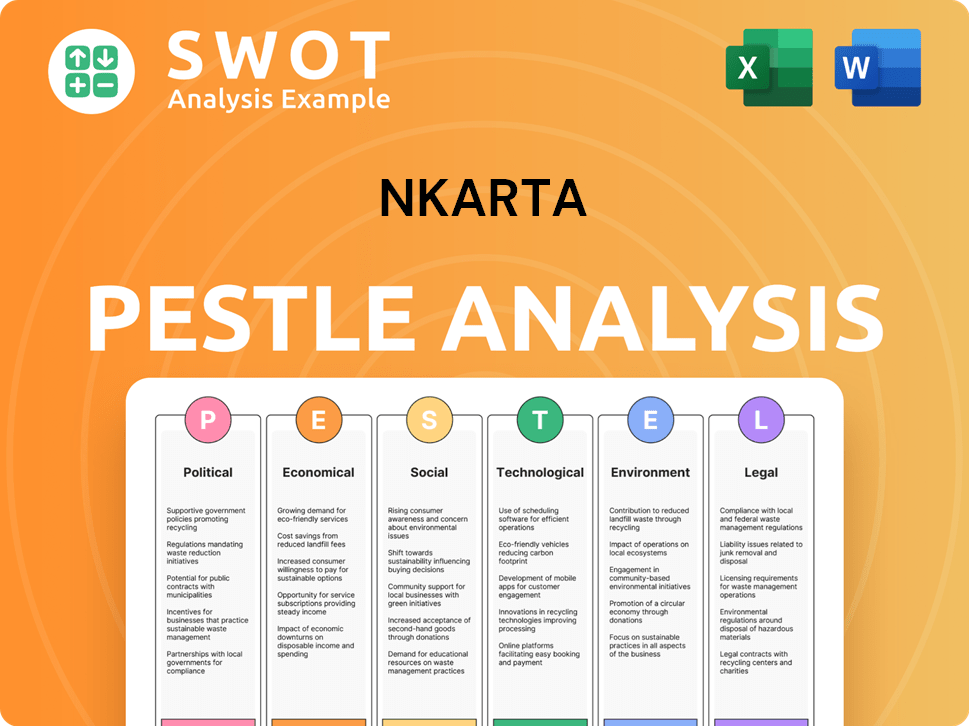
The geographical market presence of the biopharmaceutical company, is primarily defined by its operational base and the locations of its clinical trials. Based in South San Francisco, California, United States, the company's activities are currently concentrated within the U.S. due to its clinical trial locations.
The company's clinical trials for its lead product candidate, NKX019, are predominantly U.S.-based. This focus on the U.S. market is typical for clinical-stage biopharmaceutical companies, as they seek regulatory approval in key markets. The Ntrust-1 and Ntrust-2 trials, for example, are multicenter trials within the U.S.
As a clinical-stage company, the company's geographical footprint is limited to its research, development, and clinical operations. The company does not yet have commercialized products, and therefore, does not have traditional market share or brand recognition across specific countries for product sales. The company's target 'customers' are patients enrolled in clinical trials.
The company's customer demographics are currently defined by patients enrolled in clinical trials. These patients are specifically those with conditions like lupus nephritis, systemic sclerosis, idiopathic inflammatory myopathy, ANCA-associated vasculitis, and systemic lupus erythematosus. The customer profile is shaped by the inclusion/exclusion criteria of these trials.
The target market for the company is patients suffering from specific autoimmune diseases and cancers, who are eligible for and willing to participate in clinical trials. The focus is on regulatory approval in the United States. The company's Revenue Streams & Business Model of Nkarta highlights the importance of clinical trial success for future market entry.
The market analysis for the company involves assessing the prevalence of the diseases targeted by its cell therapies. This includes understanding the unmet medical needs and the competitive landscape. Current market trends indicate a growing interest in cell therapy for autoimmune diseases.
The customer acquisition strategy currently focuses on enrolling patients in clinical trials. This involves identifying and partnering with clinical trial sites, and patient recruitment through various channels. The customer needs and wants are addressed through the potential therapeutic benefits of the cell therapies.
The competitive landscape includes other companies developing cell therapies for similar indications. The company's approach to market research report involves monitoring competitor activities and assessing the potential for differentiation. The company's success depends on clinical trial outcomes and regulatory approvals.
Potential customers are patients diagnosed with the specific diseases targeted by the company's therapies, who meet the clinical trial criteria. The customer base size will depend on the success of clinical trials and subsequent commercialization. The company's ideal customer profile is a patient who is eligible and willing to participate in a clinical trial.
Product market fit is being assessed through clinical trials, to determine if the cell therapies effectively address the unmet medical needs of the target patient populations. The company aims to demonstrate the efficacy and safety of its therapies. The company's geographical target market is currently the United States.
Customer segmentation is based on disease type and severity, as well as eligibility for clinical trials. The company's focus is on patients with specific autoimmune diseases and cancers. The company's market share analysis is not applicable at this stage, as it is a clinical-stage company.
Nkarta Business Model Canvas
- Complete 9-Block Business Model Canvas
- Effortlessly Communicate Your Business Strategy
- Investor-Ready BMC Format
- 100% Editable and Customizable
- Clear and Structured Layout
How Does Nkarta Win & Keep Customers?
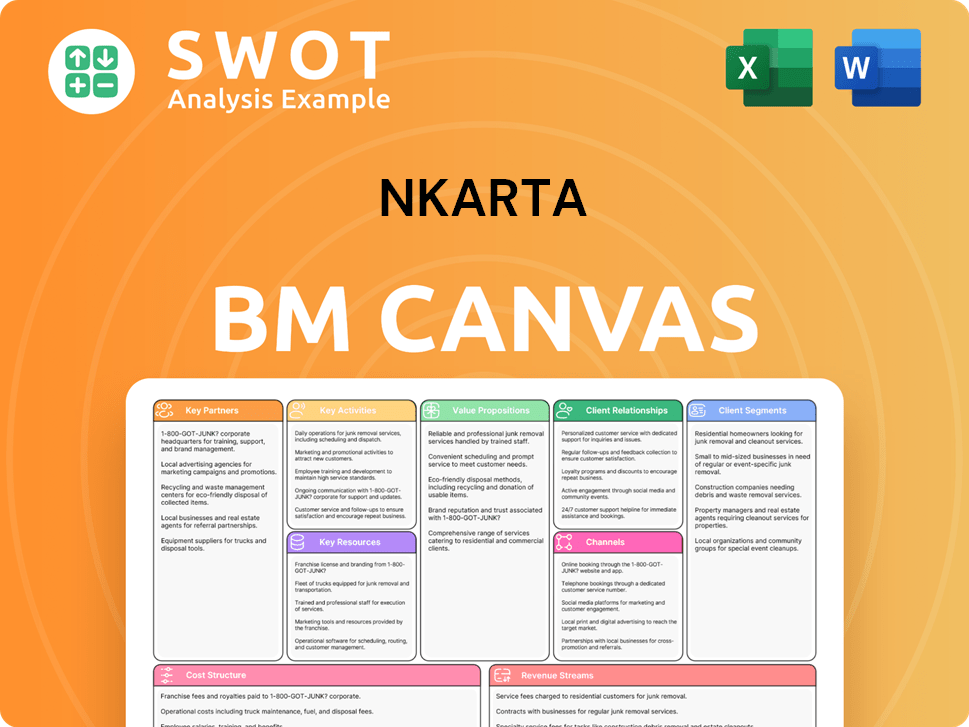
For a clinical-stage biopharmaceutical company like Nkarta, the terms 'customer demographics' and 'target market' take on a unique meaning. Instead of focusing on consumer sales, the primary focus is on patient enrollment and retention in clinical trials, as well as securing investments and partnerships. This approach is crucial for the company's survival and advancement of its cell therapy products.
The company's customer acquisition strategy centers around identifying and enrolling eligible patients for its clinical trials. This involves working closely with clinical sites and investigators to screen patients for trials such as Ntrust-1 and Ntrust-2. Furthermore, partnerships with leading researchers and institutions are vital for expanding patient access and generating additional data.
Nkarta's success hinges on its ability to attract and retain patients in its clinical trials. Key factors include the safety and tolerability of treatments, the potential for positive clinical outcomes, and the convenience of outpatient settings. These elements are vital for ensuring patient participation and adherence to trial protocols.
Nkarta initiates multi-center clinical trials like Ntrust-1 and Ntrust-2, working with clinical sites to identify eligible patients. The company actively engages with clinical investigators to screen and enroll patients into its trials. This is a crucial step in the Competitors Landscape of Nkarta's clinical development strategy.
Collaborations with leading researchers and institutions are vital for expanding patient access. These partnerships generate additional data and support the advancement of their investigational therapies. An example is the IST for systemic lupus erythematosus at Columbia University Irving Medical Center.
Nkarta targets specific autoimmune diseases, such as lupus nephritis, systemic sclerosis, myositis, and vasculitis. This approach focuses efforts on patient populations with high unmet needs. This facilitates enrollment by focusing on specific patient groups.
A key aspect of retention in clinical trials is the safety profile and tolerability of the treatment. Nkarta's NKX019 is designed to reduce toxicity, potentially improving patient experience. This includes features like fludarabine-free lymphodepletion.
The prospect of deep and durable responses to their conditions motivates patients to remain in trials. Academic research supports this, particularly in CD19-targeted cell therapy for autoimmune diseases. This motivates patients to stay in trials.
Nkarta aims for its therapies to be accessible in an outpatient setting. This could reduce the burden on patients and improve their willingness to participate in trials. Outpatient settings improve patient convenience.
Nkarta regularly reports its financial results and participates in investor conferences. The company's financial results, such as the fourth quarter and full year 2024 results showed a cash balance of $380.5 million as of December 31, 2024. They attend events like the 24th Annual Needham Virtual Healthcare Conference (April 2025) and Leerink Partners 2025 Global Healthcare Conference (March 2025).
The anticipation of preliminary clinical data from Ntrust-1 and Ntrust-2 in the second half of 2025 is a critical factor. These updates are key to attracting and retaining investor confidence. Positive data is crucial for investor confidence.
In March 2025, Nkarta implemented a restructuring plan, including a 34% workforce reduction (53 positions). This was done to extend its cash runway and prioritize clinical execution. This demonstrates a commitment to financial discipline.
The decision to discontinue NKX019 development in lymphoma to focus on autoimmune diseases is strategic. This move optimizes resources and concentrates on areas with perceived higher potential. This is communicated to stakeholders.
Nkarta's success in customer acquisition and retention is measured by patient enrollment rates, trial completion, and clinical outcomes. These factors directly impact investor confidence and the company's valuation.
- Patient Enrollment Rates: The speed at which patients are recruited for clinical trials.
- Trial Completion: The percentage of patients who finish the trial.
- Clinical Outcomes: The effectiveness and safety of the therapies.
- Regulatory Approvals: The success of the company in obtaining approvals.
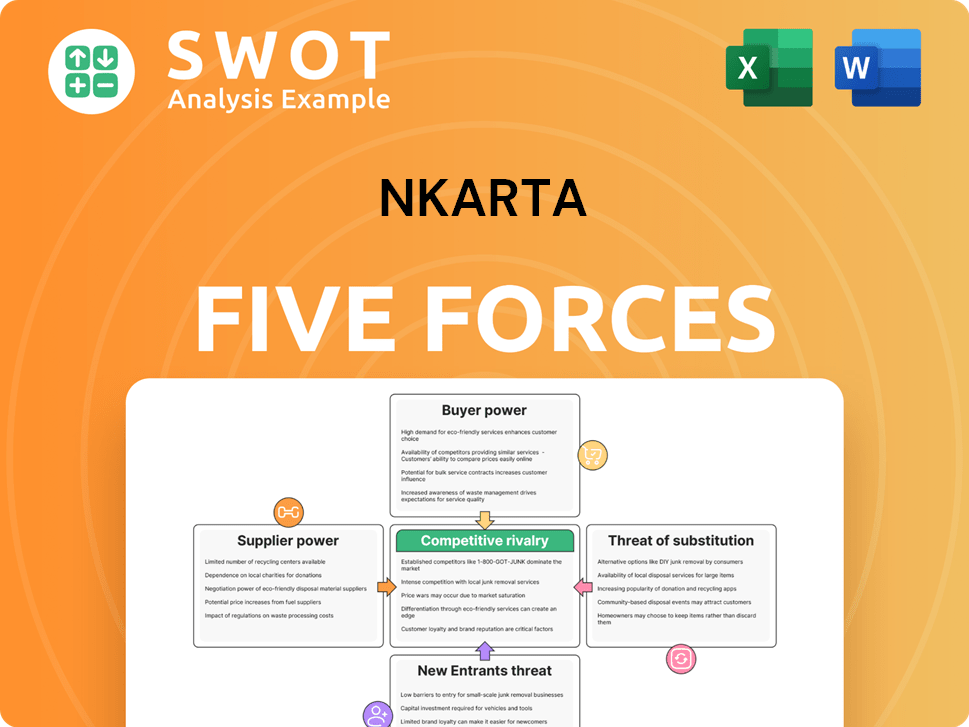
Nkarta Porter's Five Forces Analysis
- Covers All 5 Competitive Forces in Detail
- Structured for Consultants, Students, and Founders
- 100% Editable in Microsoft Word & Excel
- Instant Digital Download – Use Immediately
- Compatible with Mac & PC – Fully Unlocked
Related Blogs
- What are Mission Vision & Core Values of Nkarta Company?
- What is Competitive Landscape of Nkarta Company?
- What is Growth Strategy and Future Prospects of Nkarta Company?
- How Does Nkarta Company Work?
- What is Sales and Marketing Strategy of Nkarta Company?
- What is Brief History of Nkarta Company?
- Who Owns Nkarta Company?
Disclaimer
All information, articles, and product details provided on this website are for general informational and educational purposes only. We do not claim any ownership over, nor do we intend to infringe upon, any trademarks, copyrights, logos, brand names, or other intellectual property mentioned or depicted on this site. Such intellectual property remains the property of its respective owners, and any references here are made solely for identification or informational purposes, without implying any affiliation, endorsement, or partnership.
We make no representations or warranties, express or implied, regarding the accuracy, completeness, or suitability of any content or products presented. Nothing on this website should be construed as legal, tax, investment, financial, medical, or other professional advice. In addition, no part of this site—including articles or product references—constitutes a solicitation, recommendation, endorsement, advertisement, or offer to buy or sell any securities, franchises, or other financial instruments, particularly in jurisdictions where such activity would be unlawful.
All content is of a general nature and may not address the specific circumstances of any individual or entity. It is not a substitute for professional advice or services. Any actions you take based on the information provided here are strictly at your own risk. You accept full responsibility for any decisions or outcomes arising from your use of this website and agree to release us from any liability in connection with your use of, or reliance upon, the content or products found herein.